Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements
- PMID: 24298051
- PMCID: PMC3861665
- DOI: 10.1101/gad.229559.113
Chromothripsis and beyond: rapid genome evolution from complex chromosomal rearrangements
Abstract
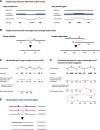
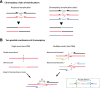
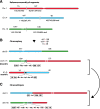
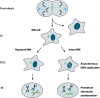
Recent genome sequencing studies have identified several classes of complex genomic rearrangements that appear to be derived from a single catastrophic event. These discoveries identify ways that genomes can be altered in single large jumps rather than by many incremental steps. Here we compare and contrast these phenomena and examine the evidence that they arise "all at once." We consider the impact of massive chromosomal change for the development of diseases such as cancer and for evolution more generally. Finally, we summarize current models for underlying mechanisms and discuss strategies for testing these models.
Keywords: cancer; chromoanasynthesis; chromoplexy; chromosomal translocation; chromothripsis; copy number alteration; genome evolution.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
