Ephrin-B2 controls PDGFRβ internalization and signaling
- PMID: 24298057
- PMCID: PMC3861671
- DOI: 10.1101/gad.224089.113
Ephrin-B2 controls PDGFRβ internalization and signaling
Erratum in
-
Corrigendum: Ephrin-B2 controls PDGFRβ internalization and signaling.Genes Dev. 2025 Nov 1;39(21-22):1395. doi: 10.1101/gad.353351.125. Genes Dev. 2025. PMID: 41184083 Free PMC article. No abstract available.
Abstract
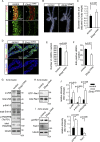
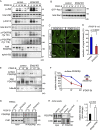
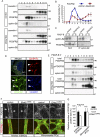
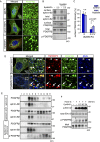
B-class ephrins, ligands for EphB receptor tyrosine kinases, are critical regulators of growth and patterning processes in many organs and species. In the endothelium of the developing vasculature, ephrin-B2 controls endothelial sprouting and proliferation, which has been linked to vascular endothelial growth factor (VEGF) receptor endocytosis and signaling. Ephrin-B2 also has essential roles in supporting mural cells (namely, pericytes and vascular smooth muscle cells [VSMCs]), but the underlying mechanism is not understood. Here, we show that ephrin-B2 controls platelet-derived growth factor receptor β (PDGFRβ) distribution in the VSMC plasma membrane, endocytosis, and signaling in a fashion that is highly distinct from its role in the endothelium. Absence of ephrin-B2 in cultured VSMCs led to the redistribution of PDGFRβ from caveolin-positive to clathrin-associated membrane fractions, enhanced PDGF-B-induced PDGFRβ internalization, and augmented downstream mitogen-activated protein (MAP) kinase and c-Jun N-terminal kinase (JNK) activation but impaired Tiam1-Rac1 signaling and proliferation. Accordingly, mutant mice lacking ephrin-B2 expression in vascular smooth muscle developed vessel wall defects and aortic aneurysms, which were associated with impaired Tiam1 expression and excessive activation of MAP kinase and JNK. Our results establish that ephrin-B2 is an important regulator of PDGFRβ endocytosis and thereby acts as a molecular switch controlling the downstream signaling activity of this receptor in mural cells.
Keywords: PDGF; receptor; signaling; tyrosine kinase.
Figures

References
-
- Abella JV, Park M 2009. Breakdown of endocytosis in the oncogenic activation of receptor tyrosine kinases. Am J Physiol Endocrinol Metab 296: E973–E984 - PubMed
-
- Batlle E, Henderson JT, Beghtel H, van den Born MM, Sancho E, Huls G, Meeldijk J, Robertson J, van de Wetering M, Pawson T, et al. 2002. β-Catenin and TCF mediate cell positioning in the intestinal epithelium by controlling the expression of EphB/ephrinB. Cell 111: 251–263 - PubMed
-
- Bond M, Wu YJ, Sala-Newby GB, Newby AC 2008. Rho GTPase, Rac1, regulates Skp2 levels, vascular smooth muscle cell proliferation, and intima formation in vitro and in vivo. Cardiovasc Res 80: 290–298 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
