Microtubule-associated protein tau is essential for long-term depression in the hippocampus
- PMID: 24298146
- PMCID: PMC3843876
- DOI: 10.1098/rstb.2013.0144
Microtubule-associated protein tau is essential for long-term depression in the hippocampus
Abstract
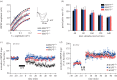
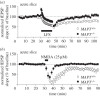
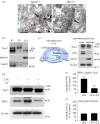
The microtubule-associated protein tau is a principal component of neurofibrillary tangles, and has been identified as a key molecule in Alzheimer's disease and other tauopathies. However, it is unknown how a protein that is primarily located in axons is involved in a disease that is believed to have a synaptic origin. To investigate a possible synaptic function of tau, we studied synaptic plasticity in the hippocampus and found a selective deficit in long-term depression (LTD) in tau knockout mice in vivo and in vitro, an effect that was replicated by RNAi knockdown of tau in vitro. We found that the induction of LTD is associated with the glycogen synthase kinase-3-mediated phosphorylation of tau. These observations demonstrate that tau has a critical physiological function in LTD.
Keywords: Alzheimer's disease; hippocampus; long-term depression; synaptic plasticity; tau.
Figures

References
-
- Andreadis A, Brown WM, Kosik KS. 1992. Structure and novel exons of the human tau gene. Biochemistry 31, 10 626–10 633 (doi:10.1021/bi00158a027) - DOI - PubMed
-
- Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. 1988. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc. Natl Acad. Sci. USA 85, 4051–4055 (doi:10.1073/pnas.85.11.4051) - DOI - PMC - PubMed
-
- Lindwall G, Cole RD. 1984. Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305 - PubMed
-
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. 1993. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron 11, 153–163 (doi:10.1016/0896-6273(93)90279-Z) - DOI - PubMed
-
- Weingarten MD, Lockwood AH, Hwo SY, Kirschner MW. 1975. A protein factor essential for microtubule assembly. Proc. Natl Acad. Sci. USA 72, 1858–1862 (doi:10.1073/pnas.72.5.1858) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
