Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis
- PMID: 24298163
- PMCID: PMC3843893
- DOI: 10.1098/rstb.2013.0162
Synaptic plasticity in multiple sclerosis and in experimental autoimmune encephalomyelitis
Abstract
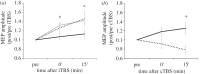
Approximately half of all patients with multiple sclerosis (MS) experience cognitive dysfunction, including learning and memory impairment. Recent studies suggest that hippocampal pathology is involved, although the mechanisms underlying these deficits remain poorly understood. Evidence obtained from a mouse model of MS, the experimental autoimmune encephalomyelitis (EAE), suggests that in the hippocampus of EAE mice long-term potentiation (LTP) is favoured over long-term depression in response to repetitive synaptic activation, through a mechanism dependent on enhanced IL-1β released from infiltrating lymphocytes or activated microglia. Facilitated LTP during an immune-mediated attack might underlie functional recovery, but also cognitive deficits and excitotoxic neurodegeneration. Having identified that pro-inflammatory cytokines such as IL-1β can influence synaptic function and integrity in early MS, it is hoped that new treatments targeted towards preventing synaptic pathology can be developed.
Keywords: experimental autoimmune encephalomyelitis; hippocampus; interleukin-1β; long-term potentiation; multiple sclerosis; synaptic plasticity.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

