The role of clomipramine in potentiating the teratogenic effects of caffeine in pregnant rats: a histopathological study
- PMID: 24298213
- PMCID: PMC3835609
- DOI: 10.1155/2013/382434
The role of clomipramine in potentiating the teratogenic effects of caffeine in pregnant rats: a histopathological study
Abstract
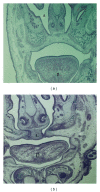
Since little is known about the teratogenic effects of clomipramine used concurrently with caffeine during the organogenesis period, the aim of this study was to test the teratogenic effects of a coadministration of caffeine and clomipramine on rat fetuses. We divided 42 pregnant rats into seven groups, randomly. The first group (control) received 0.5 mL of normal saline. Clomipramine was injected at 40 mg/kg and 80 mg/kg to the second and third groups, respectively. The fourth and fifth groups received caffeine in doses of 60 mg/kg and 120 mg/kg, respectively. The sixth group received a combination of 40 mg/kg clomipramine and 60 mg/kg caffeine, and the seventh group was given clomipramine and caffeine at 80 mg/kg and 120 mg/kg, respectively. The fetuses were removed on the 17th day of pregnancy and studied in terms of microscopic and macroscopic morphological features. Fetuses of rats receiving high doses of caffeine or combinations of caffeine and clomipramine showed a significant rate of cleft palate development, open eyelids, mortality, torsion anomalies, shrinkage of skin, and subcutaneous haemorrhage (P ≤ 0.001). This study concludes that caffeine in high doses or the simultaneous administration of caffeine and clomipramine leads to teratogenicity.
Figures


References
-
- Newman CG. The thalidomide syndrome: risks of exposure and spectrum of malformations. Clinics in Perinatology. 1986;13(3):555–573. - PubMed
-
- Swaab DF, Mirmiran M. Functional teratogenic effects of chemicals on the developing brain. Monographs in Neural Sciences. 1986;12:45–57. - PubMed
-
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. European Journal of Pharmacology. 1997;340(2-3):249–258. - PubMed
-
- Busto U, Bendayan R, Sellers EM. Clinical pharmacokinetics of non-opiate abused drugs. Clinical Pharmacokinetics. 1989;16(1):1–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

