Tissue regeneration and stem cell distribution in adriamycin induced glomerulopathy
- PMID: 24298364
- PMCID: PMC3840995
- DOI: 10.15283/ijsc.2012.5.2.115
Tissue regeneration and stem cell distribution in adriamycin induced glomerulopathy
Abstract
Background and objectives: Glomerulosclerosis develops secondary to various kidney diseases. It was postulated that adriamycin (ADR) induce chronic glomerulopathy. Treatment combinations for one year did not significantly modify renal function in resistant focal segmental glomerulosclerosis (FSGS). Recurrence of FSGS after renal transplantation impacts long-term graft survival and limits access to transplantation. The present study aimed at investigating the relation between the possible therapeutic effect of human mesenchymal stem cells (HMSCs), isolated from cord blood on glomerular damage and their distribution by using ADR induced nephrotoxicity as a model in albino rat.
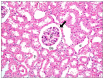
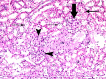
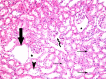
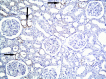
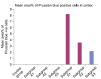
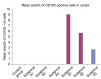
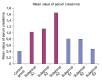
Methods and results: Thirty three male albino rats were divided into control group, ADR group where rats were given single intraperitoneal (IP) injection of 5 mg/kg adriamycin. The rats were sacrificed 10, 20 and 30 days following confirmation of glomerular injury. In stem cell therapy group, rats were injected with HMSCs following confirmation of renal injury and sacrificed 10, 20 and 30 days after HMSCs therapy. Kidney sections were exposed to histological, histochemical, immunohistochemical, morphometric and serological studies. In response to SC therapy multiple Malpighian corpuscles (MC) appeared with patent Bowman's space (Bs) 10 and 20 days following therapy. One month following therapy no remarkable shrunken glomeruli were evident. Glomerular area and serum creatinine were significantly different in ADR group in comparison to control and SC therapy groups.
Conclusions: ADR induced glomerulosclerosis regressed in response to cord blood HMSC therapy. A reciprocal relation was recorded between the extent of renal regeneration and the distribution of undifferentiated mesenchymal stem cells.
Keywords: Adriamycin; Cord blood; Glomerulosclerosis; Mesenchymal stem cells.
Figures











Similar articles
-
Effect of stem cell therapy on adriamycin induced tubulointerstitial injury.Int J Stem Cells. 2012 Nov;5(2):130-9. doi: 10.15283/ijsc.2012.5.2.130. Int J Stem Cells. 2012. PMID: 24298366 Free PMC article.
-
Histological experimental study on the effect of stem cell therapy on adriamycin induced chemobrain.Int J Stem Cells. 2013 Nov;6(2):104-12. doi: 10.15283/ijsc.2013.6.2.104. Int J Stem Cells. 2013. PMID: 24386554 Free PMC article.
-
[Slowing the progression of chronic renal insufficiency with captopril in rats with spontaneous arterial hypertension and adriamycin nephropathy].Srp Arh Celok Lek. 2002 Mar-Apr;130(3-4):73-80. doi: 10.2298/sarh0204073j. Srp Arh Celok Lek. 2002. PMID: 12154518 Serbian.
-
Focal segmental glomerulosclerosis after renal transplantation.Clin Transplant. 2011 Jul;25 Suppl 23:6-14. doi: 10.1111/j.1399-0012.2011.01452.x. Clin Transplant. 2011. PMID: 21623907 Review.
-
Pathophysiology and treatment of focal segmental glomerulosclerosis: the role of animal models.BMC Nephrol. 2013 Apr 1;14:74. doi: 10.1186/1471-2369-14-74. BMC Nephrol. 2013. PMID: 23547922 Free PMC article. Review.
Cited by
-
Regular voluntary running has favorable histological effects on doxorubicin-induced kidney toxicity in Wistar rats.Cell Tissue Res. 2018 Oct;374(1):177-187. doi: 10.1007/s00441-018-2840-z. Epub 2018 Apr 30. Cell Tissue Res. 2018. PMID: 29713815
-
Original Research: Potential of urinary nephrin as a biomarker reflecting podocyte dysfunction in various kidney disease models.Exp Biol Med (Maywood). 2016 Oct;241(16):1865-76. doi: 10.1177/1535370216651937. Epub 2016 May 22. Exp Biol Med (Maywood). 2016. PMID: 27216597 Free PMC article.
References
-
- Gautier JC, Riefke B, Walter J, Kurth P, Mylecraine L, Guilpin V, Barlow N, Gury T, Hoffman D, Ennulat D, Schuster K, Harpur E, Pettit S. Evaluation of novel biomarkers of nephrotoxicity in two strains of rat treated with Cisplatin. Toxicol Pathol. 2010;38:943–956. - PubMed
-
- Jiao YQ, Yi ZW, He XJ, Liu XH, He QN, Huang DL. Does injection of metanephric mesenchymal cells improve renal function in rats? Saudi J Kidney Dis Transpl. 2011;22:501–510. - PubMed
-
- Sasaki M, Shikata K, Okada S, Miyamoto S, Nishishita S, Kataoka HU, Sato C, Wada J, Ogawa D, Makino H. The macrophage is a key factor in renal injuries caused by glomerular hyperfiltration. Acta Med Okayama. 2011;65:81–89. - PubMed
-
- Segarra Medrano A, Vila Presas J, Pou Clavé L, Majó Masferrer J, Camps Doménech J. Efficacy and safety of combined cyclosporin A and mycophenolate mofetil therapy in patients with cyclosporin-resistant focal segmental glomerulosclerosis. Nefrologia. 2011;31:286–291. - PubMed
-
- Rivera Hernández F. How to treat corticosteroid-resistant idiopathic focal segmental glomerulosclerosis? Nefrologia. 2011;31:247–250. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

