In vivo imaging of retinal pigment epithelium cells in age related macular degeneration
- PMID: 24298413
- PMCID: PMC3829547
- DOI: 10.1364/BOE.4.002527
In vivo imaging of retinal pigment epithelium cells in age related macular degeneration
Abstract
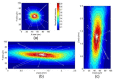
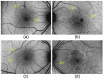
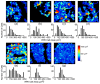
Morgan and colleagues demonstrated that the RPE cell mosaic can be resolved in the living human eye non-invasively by imaging the short-wavelength autofluorescence using an adaptive optics (AO) ophthalmoscope. This method, based on the assumption that all subjects have the same longitudinal chromatic aberration (LCA) correction, has proved difficult to use in diseased eyes, and in particular those affected by age-related macular degeneration (AMD). In this work, we improve Morgan's method by accounting for chromatic aberration variations by optimizing the confocal aperture axial and transverse placement through an automated iterative maximization of image intensity. The increase in image intensity after algorithmic aperture placement varied depending upon patient and aperture position prior to optimization but increases as large as a factor of 10 were observed. When using a confocal aperture of 3.4 Airy disks in diameter, images were obtained using retinal radiant exposures of less than 2.44 J/cm(2), which is ~22 times below the current ANSI maximum permissible exposure. RPE cell morphologies that were strikingly similar to those seen in postmortem histological studies were observed in AMD eyes, even in areas where the pattern of fluorescence appeared normal in commercial fundus autofluorescence (FAF) images. This new method can be used to study RPE morphology in AMD and other diseases, providing a powerful tool for understanding disease pathogenesis and progression, and offering a new means to assess the efficacy of treatments designed to restore RPE health.
Keywords: (110.1080) Active or adaptive optics; (170.1610) Clinical applications; (170.3880) Medical and biological imaging; (170.4470) Ophthalmology; (330.5310) Vision - photoreceptors.
Figures



References
-
- Green W. R., McDonnell P. J., Yeo J. H., “Pathologic features of senile macular degeneration,” Ophthalmology 92(5), 615–627 (1985). - PubMed
-
- Kaneko H., Dridi S., Tarallo V., Gelfand B. D., Fowler B. J., Cho W. G., Kleinman M. E., Ponicsan S. L., Hauswirth W. W., Chiodo V. A., Karikó K., Yoo J. W., Lee D. K., Hadziahmetovic M., Song Y., Misra S., Chaudhuri G., Buaas F. W., Braun R. E., Hinton D. R., Zhang Q., Grossniklaus H. E., Provis J. M., Madigan M. C., Milam A. H., Justice N. L., Albuquerque R. J. C., Blandford A. D., Bogdanovich S., Hirano Y., Witta J., Fuchs E., Littman D. R., Ambati B. K., Rudin C. M., Chong M. M. W., Provost P., Kugel J. F., Goodrich J. A., Dunaief J. L., Baffi J. Z., Ambati J., “DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration,” Nature 471(7338), 325–330 (2011).10.1038/nature09830 - DOI - PMC - PubMed
-
- Rudolf M., Vogt S. D., Curcio C. A., Huisingh C., McGwin G., Jr, Wagner A., Grisanti S., Read R. W., “Histologic basis of variations in retinal pigment epithelium autofluorescence in eyes with geographic atrophy,” Ophthalmology 120(4), 821–828 (2013).10.1016/j.ophtha.2012.10.007 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
