Mechanism of enhanced superoxide production in the cytochrome b(6)f complex of oxygenic photosynthesis
- PMID: 24298890
- PMCID: PMC4037229
- DOI: 10.1021/bi4013534
Mechanism of enhanced superoxide production in the cytochrome b(6)f complex of oxygenic photosynthesis
Abstract
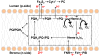
The specific rate of superoxide (O2(•-)) production in the purified active crystallizable cytochrome b6f complex, normalized to the rate of electron transport, has been found to be more than an order of magnitude greater than that measured in isolated yeast respiratory bc1 complex. The biochemical and structural basis for the enhanced production of O2(•-) in the cytochrome b6f complex compared to that in the bc1 complex is discussed. The higher rate of superoxide production in the b6f complex could be a consequence of an increased residence time of plastosemiquinone/plastoquinol in its binding niche near the Rieske protein iron-sulfur cluster, resulting from (i) occlusion of the quinone portal by the phytyl chain of the unique bound chlorophyll, (ii) an altered environment of the proton-accepting glutamate believed to be a proton acceptor from semiquinone, or (iii) a more negative redox potential of the heme bp on the electrochemically positive side of the complex. The enhanced rate of superoxide production in the b6f complex is physiologically significant as the chloroplast-generated reactive oxygen species (ROS) functions in the regulation of excess excitation energy, is a source of oxidative damage inflicted during photosynthetic reactions, and is a major source of ROS in plant cells. Altered levels of ROS production are believed to convey redox signaling from the organelle to the cytosol and nucleus.
Figures



References
-
- Cape JL, Bowman MK, Kramer DM. Understanding the cytochrome bc complexes by what they don't do, The Q-cycle at 30. Trends Plant Sci. 2006;11:46–55. - PubMed
-
- Drose S, Brandt U. The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem. 2008;283:21649–21654. - PubMed
-
- Forquer I, Covian R, Bowman MK, Trumpower B, Kramer DM. Similar transition states mediate the Q-cycle and superoxide production by the cytochrome bc1 complex. J Biol Chem. 2006;281:38459–38465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

