Relative resistance of HIV-1 founder viruses to control by interferon-alpha
- PMID: 24299076
- PMCID: PMC3907080
- DOI: 10.1186/1742-4690-10-146
Relative resistance of HIV-1 founder viruses to control by interferon-alpha
Abstract
Background: Following mucosal human immunodeficiency virus type 1 (HIV-1) transmission, type 1 interferons (IFNs) are rapidly induced at sites of initial virus replication in the mucosa and draining lymph nodes. However, the role played by IFN-stimulated antiviral activity in restricting HIV-1 replication during the initial stages of infection is not clear. We hypothesized that if type 1 IFNs exert selective pressure on HIV-1 replication in the earliest stages of infection, the founder viruses that succeed in establishing systemic infection would be more IFN-resistant than viruses replicating during chronic infection, when type 1 IFNs are produced at much lower levels. To address this hypothesis, the relative resistance of virus isolates derived from HIV-1-infected individuals during acute and chronic infection to control by type 1 IFNs was analysed.
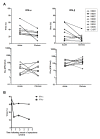
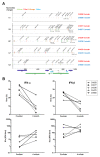
Results: The replication of plasma virus isolates generated from subjects acutely infected with HIV-1 and molecularly cloned founder HIV-1 strains could be reduced but not fully suppressed by type 1 IFNs in vitro. The mean IC50 value for IFNα2 (22 U/ml) was lower than that for IFNβ (346 U/ml), although at maximally-inhibitory concentrations both IFN subtypes inhibited virus replication to similar extents. Individual virus isolates exhibited differential susceptibility to inhibition by IFNα2 and IFNβ, likely reflecting variation in resistance to differentially up-regulated IFN-stimulated genes. Virus isolates from subjects acutely infected with HIV-1 were significantly more resistant to in vitro control by IFNα than virus isolates generated from the same individuals during chronic, asymptomatic infection. Viral IFN resistance declined rapidly after the acute phase of infection: in five subjects, viruses derived from six-month consensus molecular clones were significantly more sensitive to the antiviral effects of IFNs than the corresponding founder viruses.
Conclusions: The establishment of systemic HIV-1 infection by relatively IFNα-resistant founder viruses lends strong support to the hypothesis that IFNα plays an important role in the control of HIV-1 replication during the earliest stages of infection, prior to systemic viral spread. These findings suggest that it may be possible to harness the antiviral activity of type 1 IFNs in prophylactic and potentially also therapeutic strategies to combat HIV-1 infection.
Figures


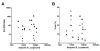

References
-
- Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H. et al.Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;10:7552–7557. doi: 10.1073/pnas.0802203105. - DOI - PMC - PubMed
-
- Abrahams MR, Anderson JA, Giorgi EE, Seoighe C, Mlisana K, Ping LH, Athreya GS, Treurnicht FK, Keele BF, Wood N. et al.Quantitating the multiplicity of infection with human immunodeficiency virus type 1 subtype C reveals a non-poisson distribution of transmitted variants. J Virol. 2009;10:3556–3567. doi: 10.1128/JVI.02132-08. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

