PDL241, a novel humanized monoclonal antibody, reveals CD319 as a therapeutic target for rheumatoid arthritis
- PMID: 24299175
- PMCID: PMC3978732
- DOI: 10.1186/ar4400
PDL241, a novel humanized monoclonal antibody, reveals CD319 as a therapeutic target for rheumatoid arthritis
Abstract
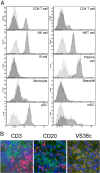
Introduction: Targeting the CD20 antigen has been a successful therapeutic intervention in the treatment of rheumatoid arthritis (RA). However, in some patients with an inadequate response to anti-CD20 therapy, a persistence of CD20- plasmablasts is noted. The strong expression of CD319 on CD20- plasmablast and plasma cell populations in RA synovium led to the investigation of the potential of CD319 as a therapeutic target.
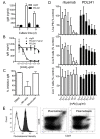
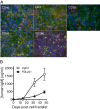
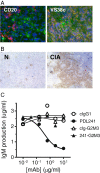
Methods: PDL241, a novel humanized IgG1 monoclonal antibody (mAb) to CD319, was generated and examined for its ability to inhibit immunoglobulin production from plasmablasts and plasma cells generated from peripheral blood mononuclear cells (PBMC) in the presence and absence of RA synovial fibroblasts (RA-SF). The in vivo activity of PDL241 was determined in a human PBMC transfer into NOD scid IL-2 gamma chain knockout (NSG) mouse model. Finally, the ability of PDL241 to ameliorate experimental arthritis was evaluated in a collagen-induced arthritis (CIA) model in rhesus monkeys.
Results: PDL241 bound to plasmablasts and plasma cells but not naïve B cells. Consistent with the binding profile, PDL241 inhibited the production of IgM from in vitro PBMC cultures by the depletion of CD319+ plasmablasts and plasma cells but not B cells. The activity of PDL241 was dependent on an intact Fc portion of the IgG1 and mediated predominantly by natural killer cells. Inhibition of IgM production was also observed in the human PBMC transfer to NSG mouse model. Treatment of rhesus monkeys in a CIA model with PDL241 led to a significant inhibition of anti-collagen IgG and IgM antibodies. A beneficial effect on joint related parameters, including bone remodeling, histopathology, and joint swelling was also observed.
Conclusions: The activity of PDL241 in both in vitro and in vivo models highlights the potential of CD319 as a therapeutic target in RA.
Figures





Similar articles
-
Integrative analysis reveals CD38 as a therapeutic target for plasma cell-rich pre-disease and established rheumatoid arthritis and systemic lupus erythematosus.Arthritis Res Ther. 2018 May 2;20(1):85. doi: 10.1186/s13075-018-1578-z. Arthritis Res Ther. 2018. PMID: 29720240 Free PMC article.
-
The programmed death 1/programmed death ligand 1 inhibitory pathway is up-regulated in rheumatoid synovium and regulates peripheral T cell responses in human and murine arthritis.Arthritis Rheum. 2010 Jul;62(7):1870-80. doi: 10.1002/art.27500. Arthritis Rheum. 2010. PMID: 20506224
-
Pathogenic role of the CXCL16-CXCR6 pathway in rheumatoid arthritis.Arthritis Rheum. 2005 Oct;52(10):3004-14. doi: 10.1002/art.21301. Arthritis Rheum. 2005. PMID: 16200580
-
The SCID mouse model: novel therapeutic targets - lessons from gene transfer.Springer Semin Immunopathol. 2003 Aug;25(1):65-78. doi: 10.1007/s00281-003-0126-2. Springer Semin Immunopathol. 2003. PMID: 12904892 Review.
-
[Rheumatoid arthritis: new developments in the pathogenesis with special reference to synovial fibroblasts].Z Rheumatol. 2001 Oct;60(5):309-18. doi: 10.1007/s003930170030. Z Rheumatol. 2001. PMID: 11759230 Review. German.
Cited by
-
Pathogenic memory plasma cells in autoimmunity.Curr Opin Immunol. 2019 Dec;61:86-91. doi: 10.1016/j.coi.2019.09.005. Epub 2019 Oct 30. Curr Opin Immunol. 2019. PMID: 31675681 Free PMC article. Review.
-
SLAMF7 regulates the inflammatory response in macrophages during polymicrobial sepsis.J Clin Invest. 2023 Mar 15;133(6):e150224. doi: 10.1172/JCI150224. J Clin Invest. 2023. PMID: 36749634 Free PMC article.
-
Nanomedicines in B cell-targeting therapies.Acta Biomater. 2022 Jan 1;137:1-19. doi: 10.1016/j.actbio.2021.10.024. Epub 2021 Oct 21. Acta Biomater. 2022. PMID: 34687954 Free PMC article. Review.
-
Enhanced SLAMF7 Homotypic Interactions by Elotuzumab Improves NK Cell Killing of Multiple Myeloma.Cancer Immunol Res. 2019 Oct;7(10):1633-1646. doi: 10.1158/2326-6066.CIR-18-0579. Epub 2019 Aug 20. Cancer Immunol Res. 2019. PMID: 31431433 Free PMC article.
-
Clinical efficacy of a new CD28-targeting antagonist of T cell co-stimulation in a non-human primate model of collagen-induced arthritis.Clin Exp Immunol. 2016 Mar;183(3):405-18. doi: 10.1111/cei.12739. Epub 2015 Dec 16. Clin Exp Immunol. 2016. PMID: 26540618 Free PMC article.
References
-
- Genovese MC, Becker JC, Schiff M, Luggen M, Sherrer Y, Kremer J, Birbara C, Box J, Natarajan K, Nuamah I, Li T, Aranda R, Hagerty DT, Dougados M. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;15:1114–1123. doi: 10.1056/NEJMoa050524. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

