The ReSPonD trial--rivastigmine to stabilise gait in Parkinson's disease a phase II, randomised, double blind, placebo controlled trial to evaluate the effect of rivastigmine on gait in patients with Parkinson's disease who have fallen
- PMID: 24299497
- PMCID: PMC3880104
- DOI: 10.1186/1471-2377-13-188
The ReSPonD trial--rivastigmine to stabilise gait in Parkinson's disease a phase II, randomised, double blind, placebo controlled trial to evaluate the effect of rivastigmine on gait in patients with Parkinson's disease who have fallen
Abstract
Background: Gait impairment is common in people with Parkinson's disease. There is a lack of effective interventions to target this debilitating complication and therefore a need to identify new therapeutic options. An underlying cholinergic deficit contributes to both the gait and cognitive dysfunction seen in Parkinson's disease. The combined impact of both impairments can be assessed in gait tasks performed with concomitant cognitive tasks. The aim of this trial is to evaluate the impact of a cholinesterase inhibitor on cognitive function and gait performance in people with established Parkinson's disease.
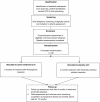
Methods/design: This is a single centre, double-blind, randomised placebo-controlled trial in 130 people with Hoehn and Yahr stage 2-3 idiopathic Parkinson's disease who have fallen in the past year. Participants will be randomised to two groups, receiving either rivastigmine capsules or identical placebo capsules for 8 months. Assessment will be undertaken at baseline and at the end of medication prescription (i.e. 8 months) with participants remaining enrolled in the trial for a further 4 months to monitor for falls and adverse events. The primary outcome is step time variability, assessed with and without the addition of concurrent cognitive tasks. Secondary outcomes will include other gait parameters, sensorimotor and balance performances, cognitive indices, falls and fall related injury, fear of falling, Parkinson's symptoms and data pertaining to possible harms.
Discussion: This randomised controlled trial will examine the effect of cholinesterase inhibitor therapy on gait, balance and falls in Parkinson's disease. If effective, it would offer a new therapeutic option to ameliorating gait and cognitive deficits in a population at high risk of falls.
Trial registration: ISRCTN19880883, UTN U1111-1124-0244.
Figures
Similar articles
-
Cholinesterase inhibitor to prevent falls in Parkinson's disease (CHIEF-PD) trial: a phase 3 randomised, double-blind placebo-controlled trial of rivastigmine to prevent falls in Parkinson's disease.BMC Neurol. 2021 Oct 29;21(1):422. doi: 10.1186/s12883-021-02430-2. BMC Neurol. 2021. PMID: 34715821 Free PMC article. Clinical Trial.
-
Rivastigmine for gait stability in patients with Parkinson's disease (ReSPonD): a randomised, double-blind, placebo-controlled, phase 2 trial.Lancet Neurol. 2016 Mar;15(3):249-58. doi: 10.1016/S1474-4422(15)00389-0. Epub 2016 Jan 13. Lancet Neurol. 2016. PMID: 26795874 Clinical Trial.
-
Exercise- and strategy-based physiotherapy-delivered intervention for preventing repeat falls in people with Parkinson's: the PDSAFE RCT.Health Technol Assess. 2019 Jul;23(36):1-150. doi: 10.3310/hta23360. Health Technol Assess. 2019. PMID: 31339100 Free PMC article. Clinical Trial.
-
Rivastigmine: in Parkinson's disease dementia.CNS Drugs. 2006;20(9):739-47; discussion 748-50. doi: 10.2165/00023210-200620090-00003. CNS Drugs. 2006. PMID: 16953649 Review.
-
Rivastigmine tartrate with a focus on dementia associated with Parkinson's disease.Drugs Today (Barc). 2007 Jun;43(6):349-59. doi: 10.1358/dot.2007.43.6.1107987. Drugs Today (Barc). 2007. PMID: 17612707 Review.
Cited by
-
Comparison of Test Your Memory and Montreal Cognitive Assessment Measures in Parkinson's Disease.Parkinsons Dis. 2016;2016:1012847. doi: 10.1155/2016/1012847. Epub 2016 Jul 5. Parkinsons Dis. 2016. PMID: 27478678 Free PMC article.
-
Interventions for preventing falls in Parkinson's disease.Cochrane Database Syst Rev. 2022 Jun 6;6(6):CD011574. doi: 10.1002/14651858.CD011574.pub2. Cochrane Database Syst Rev. 2022. PMID: 35665915 Free PMC article.
-
Effect of rivastigmine on mobility of patients with higher-level gait disorder: a pilot exploratory study.Drugs R D. 2014 Jun;14(2):57-62. doi: 10.1007/s40268-014-0038-8. Drugs R D. 2014. PMID: 24723147 Free PMC article. Clinical Trial.
-
Pharmacological treatment in Parkinson's disease: Effects on gait.Parkinsonism Relat Disord. 2016 Oct;31:3-13. doi: 10.1016/j.parkreldis.2016.07.006. Epub 2016 Jul 17. Parkinsonism Relat Disord. 2016. PMID: 27461783 Free PMC article. Review.
-
Gait Disorder in a Cohort of Patients With Mild and Moderate Alzheimer's Disease.Am J Alzheimers Dis Other Demen. 2016 May;31(3):257-62. doi: 10.1177/1533317515603113. Epub 2015 Sep 22. Am J Alzheimers Dis Other Demen. 2016. PMID: 26395024 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous