Xenon and other volatile anesthetics change domain structure in model lipid raft membranes
- PMID: 24299622
- PMCID: PMC3914297
- DOI: 10.1021/jp411261g
Xenon and other volatile anesthetics change domain structure in model lipid raft membranes
Abstract
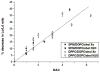
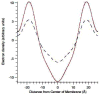
Inhalation anesthetics have been in clinical use for over 160 years, but the molecular mechanisms of action continue to be investigated. Direct interactions with ion channels received much attention after it was found that anesthetics do not change the structure of homogeneous model membranes. However, it was recently found that halothane, a prototypical anesthetic, changes domain structure of a binary lipid membrane. The noble gas xenon is an excellent anesthetic and provides a pivotal test of the generality of this finding, extended to ternary lipid raft mixtures. We report that xenon and conventional anesthetics change the domain equilibrium in two canonical ternary lipid raft mixtures. These findings demonstrate a membrane-mediated mechanism whereby inhalation anesthetics can affect the lipid environment of transmembrane proteins.
Figures



References
-
- Sanders RD, Franks NP, Maze M. Xenon: No Stranger To Anaesthesia. Br J Anaesth. 2003;91:709–17. - PubMed
-
- Franks NP, Dickinson R, de Sousa SL, Hall AC, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. - PubMed
-
- Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, Valenzuela CA, Maze M, Franks NP. Competitive Inhibition At The Glycine Site Of The N-Methyl-D-Aspartate Receptor By The Anesthetics Xenon And Isoflurane: Evidence From Molecular Modeling And Electrophysiology. Anesthesiology. 2007;107:756–67. - PubMed
-
- Gruss M, Bushell TJ, Bright DP, Lieb WR, Mathie A, Franks NP. Two-Pore-Domain K+ Channels Are A Novel Target For The Anesthetic Gases Xenon, Nitrous Oxide, And Cyclopropane. Mol Pharmacol. 2004;65:443–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

