Advances in Lipid Nanoparticles for siRNA Delivery
- PMID: 24300520
- PMCID: PMC3836621
- DOI: 10.3390/pharmaceutics5030498
Advances in Lipid Nanoparticles for siRNA Delivery
Abstract
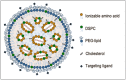
Technological advances in both siRNA (small interfering RNA) and whole genome sequencing have demonstrated great potential in translating genetic information into siRNA-based drugs to halt the synthesis of most disease-causing proteins. Despite its powerful promises as a drug, siRNA requires a sophisticated delivery vehicle because of its rapid degradation in the circulation, inefficient accumulation in target tissues and inability to cross cell membranes to access the cytoplasm where it functions. Lipid nanoparticle (LNP) containing ionizable amino lipids is the leading delivery technology for siRNA, with five products in clinical trials and more in the pipeline. Here, we focus on the technological advances behind these potent systems for siRNA-mediated gene silencing.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

