Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy
- PMID: 24301518
- PMCID: PMC3863900
- DOI: 10.1038/ncomms3890
Structural analysis and mapping of individual protein complexes by infrared nanospectroscopy
Abstract
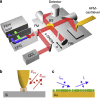
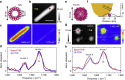
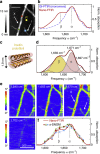
Mid-infrared spectroscopy is a widely used tool for material identification and secondary structure analysis in chemistry, biology and biochemistry. However, the diffraction limit prevents nanoscale protein studies. Here we introduce mapping of protein structure with 30 nm lateral resolution and sensitivity to individual protein complexes by Fourier transform infrared nanospectroscopy (nano-FTIR). We present local broadband spectra of one virus, ferritin complexes, purple membranes and insulin aggregates, which can be interpreted in terms of their α-helical and/or β-sheet structure. Applying nano-FTIR for studying insulin fibrils--a model system widely used in neurodegenerative disease research--we find clear evidence that 3-nm-thin amyloid-like fibrils contain a large amount of α-helical structure. This reveals the surprisingly high level of protein organization in the fibril's periphery, which might explain why fibrils associate. We envision a wide application potential of nano-FTIR, including cellular receptor in vitro mapping and analysis of proteins within quaternary structures.
Conflict of interest statement
R.H. is co-founder of Neaspec GmbH, a company producing scattering-type scanning near-field optical microscope systems, such as the one used in this study. The remaining authors declare no competing financial interests.
Figures



References
-
- Venyaminov S. Y. & Kalnin N. N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectral parameters of amino-acid residue absorption-bands. Biopolymers 30, 1243–1257 (1990). - PubMed
-
- Jackson M. & Mantsch H. H. The use and misuse of FTIR spectroscopy in the determination of protein-structure. Crit. Rev. Biochem. Mol. Biol. 30, 95–120 (1995). - PubMed
-
- Garczarek F. & Gerwert K. Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 439, 109–112 (2006). - PubMed
-
- Ataka K., Kottke T. & Heberle J. Thinner, smaller, faster: IR techniques to probe the functionality of biological and biomimetic systems. Angew. Chem Int. Ed. 49, 5416–5424 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

