Sulfation of 25-hydroxycholesterol regulates lipid metabolism, inflammatory responses, and cell proliferation
- PMID: 24302009
- PMCID: PMC3920008
- DOI: 10.1152/ajpendo.00552.2013
Sulfation of 25-hydroxycholesterol regulates lipid metabolism, inflammatory responses, and cell proliferation
Abstract
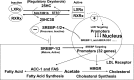
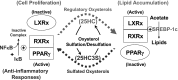
Intracellular lipid accumulation, inflammatory responses, and subsequent apoptosis are the major pathogenic events of metabolic disorders, including atherosclerosis and nonalcoholic fatty liver diseases. Recently, a novel regulatory oxysterol, 5-cholesten-3b, 25-diol 3-sulfate (25HC3S), has been identified, and hydroxysterol sulfotransferase 2B1b (SULT2B1b) has been elucidated as the key enzyme for its biosynthesis from 25-hydroxycholesterol (25HC) via oxysterol sulfation. The product 25HC3S and the substrate 25HC have been shown to coordinately regulate lipid metabolism, inflammatory responses, and cell proliferation in vitro and in vivo. 25HC3S decreases levels of the nuclear liver oxysterol receptor (LXR) and sterol regulatory element-binding proteins (SREBPs), inhibits SREBP processing, subsequently downregulates key enzymes in lipid biosynthesis, decreases intracellular lipid levels in hepatocytes and THP-1-derived macrophages, prevents apoptosis, and promotes cell proliferation in liver tissues. Furthermore, 25HC3S increases nuclear PPARγ and cytosolic IκBα and decreases nuclear NF-κB levels and proinflammatory cytokine expression and secretion when cells are challenged with LPS and TNFα. In contrast to 25HC3S, 25HC, a known LXR ligand, increases nuclear LXR and decreases nuclear PPARs and cytosol IκBα levels. In this review, we summarize our recent findings, including the discovery of the regulatory oxysterol sulfate, its biosynthetic pathway, and its functional mechanism. We also propose that oxysterol sulfation functions as a regulatory signaling pathway.
Keywords: 25HC3S, LXR, SREBPs, IκB, NF-κB, PPARs; cholesterol and triglyceride metabolism; nuclear receptor; oxysterol sulfate.
Figures


Similar articles
-
Cholesterol Metabolites 25-Hydroxycholesterol and 25-Hydroxycholesterol 3-Sulfate Are Potent Paired Regulators: From Discovery to Clinical Usage.Metabolites. 2020 Dec 25;11(1):9. doi: 10.3390/metabo11010009. Metabolites. 2020. PMID: 33375700 Free PMC article. Review.
-
25-Hydroxycholesterol-3-sulfate regulates macrophage lipid metabolism via the LXR/SREBP-1 signaling pathway.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1369-79. doi: 10.1152/ajpendo.90555.2008. Epub 2008 Oct 14. Am J Physiol Endocrinol Metab. 2008. PMID: 18854425 Free PMC article.
-
Sulfation of 25-hydroxycholesterol by SULT2B1b decreases cellular lipids via the LXR/SREBP-1c signaling pathway in human aortic endothelial cells.Atherosclerosis. 2011 Feb;214(2):350-6. doi: 10.1016/j.atherosclerosis.2010.11.021. Epub 2010 Nov 26. Atherosclerosis. 2011. PMID: 21146170 Free PMC article.
-
Oxysterol sulfation by cytosolic sulfotransferase suppresses liver X receptor/sterol regulatory element binding protein-1c signaling pathway and reduces serum and hepatic lipids in mouse models of nonalcoholic fatty liver disease.Metabolism. 2012 Jun;61(6):836-45. doi: 10.1016/j.metabol.2011.11.014. Epub 2012 Jan 5. Metabolism. 2012. PMID: 22225954 Free PMC article.
-
Action mechanisms of Liver X Receptors.Biochem Biophys Res Commun. 2014 Apr 11;446(3):647-50. doi: 10.1016/j.bbrc.2013.11.077. Epub 2013 Dec 1. Biochem Biophys Res Commun. 2014. PMID: 24300092 Review.
Cited by
-
Mutations in SULT2B1 Cause Autosomal-Recessive Congenital Ichthyosis in Humans.Am J Hum Genet. 2017 Jun 1;100(6):926-939. doi: 10.1016/j.ajhg.2017.05.007. Am J Hum Genet. 2017. PMID: 28575648 Free PMC article.
-
Cholesterol Metabolites 25-Hydroxycholesterol and 25-Hydroxycholesterol 3-Sulfate Are Potent Paired Regulators: From Discovery to Clinical Usage.Metabolites. 2020 Dec 25;11(1):9. doi: 10.3390/metabo11010009. Metabolites. 2020. PMID: 33375700 Free PMC article. Review.
-
Cholestenoic acid as endogenous epigenetic regulator decreases hepatocyte lipid accumulation in vitro and in vivo.Am J Physiol Gastrointest Liver Physiol. 2024 Feb 1;326(2):G147-G162. doi: 10.1152/ajpgi.00184.2023. Epub 2023 Nov 14. Am J Physiol Gastrointest Liver Physiol. 2024. PMID: 37961761 Free PMC article.
-
25-Hydroxycholesterol in health and diseases.J Lipid Res. 2024 Jan;65(1):100486. doi: 10.1016/j.jlr.2023.100486. Epub 2023 Dec 16. J Lipid Res. 2024. PMID: 38104944 Free PMC article. Review.
-
High levels of oxysterol sulfates in serum of patients with steroid sulfatase deficiency.J Lipid Res. 2015 Feb;56(2):403-12. doi: 10.1194/jlr.M055608. Epub 2014 Dec 11. J Lipid Res. 2015. PMID: 25502769 Free PMC article.
References
-
- Accad M, Farese RJ. Cholesterol homeostasis: a role for oxysterols. Curr Biol 8: R601–R604, 1998 - PubMed
-
- Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem 279: 52772–52780, 2004 - PubMed
-
- Alberti S, Steffensen KR, Gustafsson JA. Structural characterisation of the mouse nuclear oxysterol receptor genes LXRalpha and LXRbeta. Gene 243: 93–103, 2000 - PubMed
-
- An J, Nakajima T, Kuba K, Kimura A. Losartan inhibits LPS-induced inflammatory signaling through a PPARgamma-dependent mechanism in human THP-1 macrophages. Hypertens Res 33: 831–835, 2010 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

