Integrating pharmacogenetic information and clinical decision support into the electronic health record
- PMID: 24302286
- PMCID: PMC3994859
- DOI: 10.1136/amiajnl-2013-001873
Integrating pharmacogenetic information and clinical decision support into the electronic health record
Abstract
Pharmacogenetics (PG) examines gene variations for drug disposition, response, or toxicity. At the National Institutes of Health Clinical Center (NIH CC), a multidepartment Pharmacogenetics Testing Implementation Committee (PGTIC) was established to develop clinical decision support (CDS) algorithms for abacavir, carbamazepine, and allopurinol, medications for which human leukocyte antigen (HLA) variants predict severe hypersensitivity reactions. Providing PG CDS in the electronic health record (EHR) during order entry could prevent adverse drug events. Medical Logic Module (MLM) programming was used to implement PG CDS in our EHR. The MLM checks to see if an HLA sequence-based gene test is ordered. A message regarding test status (result present, absent, pending, or test not ordered) is displayed on the order form, and the MLM determines if the prescriber can place the order, place it but require an over-ride reason, or be blocked from placing the order. Since implementation, more than 725 medication orders have been placed for over 230 patients by 154 different prescribers for the three drugs included in our PG program. Prescribers commonly used an over-ride reason when placing the order mainly because patients had been receiving the drug without reaction before implementation of the CDS program. Successful incorporation of PG CDS into the NIH CC EHR required a coordinated, interdisciplinary effort to ensure smooth activation and a positive effect on patient care. Prescribers have adapted to using the CDS and have ordered PG testing as a direct result of the implementation.
Keywords: Clinical Decision Support; Computerized Prescriber Order Entry; Informatics; Patient Safety; Pharmacogenetics.
Figures
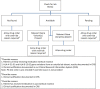

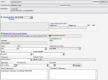
References
-
- Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med 2010;363:301–4 - PubMed
-
- Table of Pharmacogenomic Biomarkers in Drug Labels. US Food and Drug Administration. http://www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/... (accessed 1 Apr 2013).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

