Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB
- PMID: 24302727
- PMCID: PMC3894361
- DOI: 10.1074/jbc.M113.505578
Cross-talk between aryl hydrocarbon receptor and the inflammatory response: a role for nuclear factor-κB
Abstract
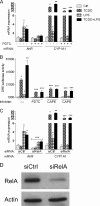
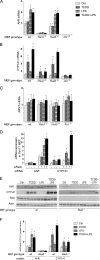
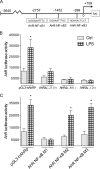
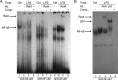
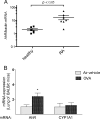
The aryl hydrocarbon receptor (AhR) is involved in the regulation of immune responses, T-cell differentiation, and immunity. Here, we show that inflammatory stimuli such as LPS induce the expression of AhR in human dendritic cells (DC) associated with an AhR-dependent increase of CYP1A1 (cytochrome P4501A1). In vivo data confirmed the elevated expression of AhR by LPS and the LPS-enhanced 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-mediated induction of CYP1A1 in thymus of B6 mice. Inhibition of nuclear factor-κB (NF-κB) repressed both normal and LPS-enhanced, TCDD-inducible, AhR-dependent gene expression and canonical pathway control of RelA-regulated AhR-responsive gene expression. LPS-mediated induction of AhR was NF-κB-dependent, as shown in mouse embryonic fibroblasts (MEFs) derived from Rel null mice. AhR expression and TCDD-mediated induction of CYP1A1 was significantly reduced in RelA-deficient MEF compared with wild type MEF cells and ectopic expression of RelA restored the expression of AhR and induction of CYP1A1 in MEF RelA null cells. Promoter analysis of the human AhR gene identified three putative NF-κB-binding elements upstream of the transcription start site. Mutation analysis of the AhR promoter identified one NF-κB site as responsible for mediating the induction of AhR expression by LPS and electrophoretic shift assays demonstrated that this NF-κB motif is recognized by the RelA/p50 heterodimer. Our results show for the first time that NF-κB RelA is a critical component regulating the expression of AhR and the induction of AhR-dependent gene expression in immune cells illustrating the interaction of AhR and NF-κB signaling.
Keywords: Aryl Hydrocarbon Receptor; Dendritic Cells; Gene Regulation; Inflammation; NF-Kappa B (NF-KB).
Figures

References
-
- Schmidt J. V., Carver L. A., Bradfield C. A. (1993) Molecular characterization of the murine Ahr gene. Organization, promoter analysis, and chromosomal assignment. J. Biol. Chem. 268, 22203–22209 - PubMed
-
- Fitzgerald C. T., Nebert D. W., Puga A. (1998) Regulation of mouse Ah receptor (Ahr) gene basal expression by members of the Sp family of transcription factors. DNA Cell. Biol. 17, 811–822 - PubMed
-
- FitzGerald C. T., Fernandez-Salguero P., Gonzalez F. J., Nebert D. W., Puga A. (1996) Differential regulation of mouse Ah receptor gene expression in cell lines of different tissue origins. Arch. Biochem. Biophys. 333, 170–178 - PubMed
-
- Ruby C. E., Leid M., Kerkvliet N. I. (2002) 2,3,7,8-Tetrachlorodibenzo-p-dioxin suppresses tumor necrosis factor-α and anti-CD40-induced activation of NF-κB/Rel in dendritic cells: p50 homodimer activation is not affected. Mol. Pharmacol. 62, 722–728 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

