KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules
- PMID: 24302764
- PMCID: PMC3870700
- DOI: 10.1073/pnas.1312637110
KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules
Abstract
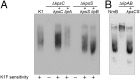
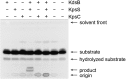
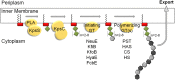
Capsular polysaccharides (CPSs) are high-molecular-mass cell-surface polysaccharides, that act as important virulence factors for many pathogenic bacteria. Several clinically important Gram-negative pathogens share similar systems for CPS biosynthesis and export; examples include Escherichia coli, Campylobacter jejuni, Haemophilus influenzae, Neisseria meningitidis, and Pasteurella multocida. Each CPS contains a serotype-specific repeat-unit structure, but the glycans all possess a lipid moiety at their reducing termini. In E. coli and N. meningitidis, the predominant lipid is a lysophosphatidylglycerol moiety that is attached to the repeat-unit domain of the CPS via multiple residues of 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo), referred to as a poly-Kdo linker. The Kdo residues are β-linked, suggesting that they are synthesized by retaining glycosyltransferases. To date, the only characterized Kdo transferases are the inverting enzymes that catalyze the α-linkages found in lipopolysaccharide. Here, we identify two conserved proteins from CPS assembly systems, KpsC and KpsS, as the β-Kdo-transferases and demonstrate in vitro reconstitution of poly-Kdo linker assembly on a fluorescent phosphatidylglycerol acceptor. KpsS adds the first Kdo residue, and this reaction product is then extended by KpsC. Cross-complementation experiments demonstrate that the E. coli and N. meningitidis protein homologs are functionally conserved.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem. 2006;75:39–68. - PubMed
-
- Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013;378:35–44. - PubMed
-
- McGuire EJ, Binkley SB. The structure and chemistry of colominic acid. Biochemistry. 1964;3:247–251. - PubMed
-
- Silver RP, et al. Molecular cloning of the K1 capsular polysaccharide genes of E. coli. Nature. 1981;289(5799):696–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

