Incomplete cytokinesis and re-fusion of small mononucleated Hodgkin cells lead to giant multinucleated Reed-Sternberg cells
- PMID: 24302766
- PMCID: PMC3870723
- DOI: 10.1073/pnas.1312509110
Incomplete cytokinesis and re-fusion of small mononucleated Hodgkin cells lead to giant multinucleated Reed-Sternberg cells
Abstract
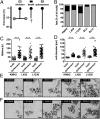
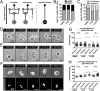
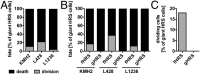
Multinucleated Reed-Sternberg (RS) cells are pathognomonic for classical Hodgkin lymphoma (HL), and their presence is essential for diagnosis. How these giant tumor cells develop is controversial, however. It has been postulated that RS cells arise from mononucleated Hodgkin cells via endomitosis. Conversely, continuous single-cell tracking of HL cell lines by long-term time-lapse microscopy has identified cell fusion as the main route of RS cell formation. In contrast to growth-induced formation of giant Hodgkin cells, fusion of small mononuclear cells followed by a size increase gives rise to giant RS cells. Of note, fusion of cells originating from the same ancestor, termed re-fusion, is seen nearly exclusively. In the majority of cases, re-fusion of daughter cells is preceded by incomplete cytokinesis, as demonstrated by microtubule bonds among the cells. We confirm at the level of individual tracked cells that giant Hodgkin and RS cells have little proliferative capacity, further supporting small mononuclear Hodgkin cells as the proliferative compartment of the HL tumor clone. In addition, sister cells show a shared propensity for re-fusion, providing evidence of early RS cell fate commitment. Thus, RS cell generation is related neither to cell fusion of unrelated Hodgkin cells nor to endomitosis, but rather is mediated by re-fusion of daughter cells that underwent mitosis. This surprising finding supports the existence of a unique mechanism for the generation of multinuclear RS cells that may have implications beyond HL, given that RS-like cells are frequently observed in several other lymphoproliferative diseases as well.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Drexler HG. Recent results on the biology of Hodgkin and Reed-Sternberg cells, I: Biopsy material. Leuk Lymphoma. 1992;8(4-5):283–313. - PubMed
-
- Sternberg C. Über eine eigenartige unter dem bilde der pseudoleukämie verlaufende tuberkulose des lymphatischen apparates. Zeitschrift für Heilkunde. 1898;19:21–90.
-
- Reed DM. On the pathological changes in Hodgkin’s disease, with special reference to its relation to tuberculosis. Johns Hopkins Hosp Rep. 1902;10:133–196.
-
- Küppers R, Schwering I, Bräuninger A, Rajewsky K, Hansmann ML. Biology of Hodgkin’s lymphoma. Ann Oncol. 2002;13(Suppl 1):11–18. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

