Rheological characterization of an acetaminophen jelly
- PMID: 24302798
- PMCID: PMC3831725
- DOI: 10.4103/0250-474X.119825
Rheological characterization of an acetaminophen jelly
Abstract
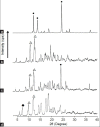
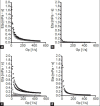
The aim of this study was to prepare an inclusion complex of acetaminophen and β-cyclodextrin (molar ratio of 1:1). A jelly with inclusion complexes formed by kneading was prepared. The formation of inclusion complexes was assessed by powder X-ray diffraction patterns and Fourier transform-infrared spectroscopy. Jellies were prepared with xanthan gum, gelatin, and κ-carrageenan. The concentration of each jelling agent was 0.5, 1.0, and 1.5% w/v. Viscoelasticity and dissolution characteristics were determined and osmometry was performed. PGWater(™), a commercial jelly for fluid replacement, served as a reference for viscoelastic characteristics and dissolution. Powder X-ray diffraction measurement revealed a different diffraction pattern for the kneading than for acetaminophen and β-cyclodextrin. Fourier transform-infrared spectroscopy revealed an absorption peak (at around 1655 cm(-1)) due to the carbonyl group and benzene ring (at around 1610 cm(-1)) of acetaminophen. In contrast, the kneaded mixture (1:1) had a shift in the absorption peak due to the carbonyl group (at around 1650 cm(-1)) in acetaminophen's molecular structure, and the formation of an inclusion complex was noted. The viscosity of xanthan gum-1.0, gelatin-1.5, and carrageenan-0.5 resembled the viscoelasticity of PGWater(™). The acetaminophen in gelatin-1.0 and carrageenan-0.5 had dissolution behavior similar to that of commercial acetaminophen preparations. The osmolality of jellies prepared in different concentrations ranged from about 20-50 mOsm/kg. Results suggested that carrageenan-0.5 could serve as a useful jelly vehicle for acetaminophen.
Keywords: Acetaminophen; jelly; viscoelasticity; β-cyclodextrin; κ-carrageenan.
Figures





References
-
- Trabal J, Leyes P, Hervás S. Factors associated with nosocomial diarrhea in patients with enteral tube feeding. Nutr Hosp. 2008;23:500–4. - PubMed
-
- Miyazaki S, Ishitani M, Takahashi A. Carrageenan gels for oral sustained delivery of acetaminophen to dysphagic patients. Biol Pharm Bull. 2011;34:164–6. - PubMed
-
- Sullivan JE, Frick GS, Maxwell LG. Fever and antipyretic use in children. Pediatrics. 2011;127:580–7. - PubMed
-
- McDaid C, Maund E, Rice S. Paracetamol and selective and nonselective nonsteroidal antiinflammatory drugs (NSAIDs) for the reduction of morphine-related side effects after major surgery. Health Technol Assess. 2010;14:1–153. - PubMed
-
- Kokki H. Nonsteroidal antiinflammatory drugs for postoperative pain: A focus on children. Paediatr Drugs. 2003;5:103–23. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
