The influence of N-linked glycans on the molecular dynamics of the HIV-1 gp120 V3 loop
- PMID: 24303005
- PMCID: PMC3841175
- DOI: 10.1371/journal.pone.0080301
The influence of N-linked glycans on the molecular dynamics of the HIV-1 gp120 V3 loop
Abstract
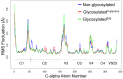
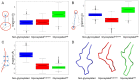
N-linked glycans attached to specific amino acids of the gp120 envelope trimer of a HIV virion can modulate the binding affinity of gp120 to CD4, influence coreceptor tropism, and play an important role in neutralising antibody responses. Because of the challenges associated with crystallising fully glycosylated proteins, most structural investigations have focused on describing the features of a non-glycosylated HIV-1 gp120 protein. Here, we use a computational approach to determine the influence of N-linked glycans on the dynamics of the HIV-1 gp120 protein and, in particular, the V3 loop. We compare the conformational dynamics of a non-glycosylated gp120 structure to that of two glycosylated gp120 structures, one with a single, and a second with five, covalently linked high-mannose glycans. Our findings provide a clear illustration of the significant effect that N-linked glycosylation has on the temporal and spatial properties of the underlying protein structure. We find that glycans surrounding the V3 loop modulate its dynamics, conferring to the loop a marked propensity towards a more narrow conformation relative to its non-glycosylated counterpart. The conformational effect on the V3 loop provides further support for the suggestion that N-linked glycosylation plays a role in determining HIV-1 coreceptor tropism.
Conflict of interest statement
Figures


Similar articles
-
Protection of neutralization epitopes in the V3 loop of oligomeric human immunodeficiency virus type 1 glycoprotein 120 by N-linked oligosaccharides in the V1 region.AIDS Res Hum Retroviruses. 2001 Jul 20;17(11):1067-76. doi: 10.1089/088922201300343753. AIDS Res Hum Retroviruses. 2001. PMID: 11485624
-
Structural dynamics of HIV-1 envelope Gp120 outer domain with V3 loop.PLoS One. 2012;7(5):e37530. doi: 10.1371/journal.pone.0037530. Epub 2012 May 18. PLoS One. 2012. PMID: 22624045 Free PMC article.
-
Identification of an N-linked glycan in the V1-loop of HIV-1 gp120 influencing neutralization by anti-V3 antibodies and soluble CD4.Arch Virol. 1994;139(3-4):253-61. doi: 10.1007/BF01310789. Arch Virol. 1994. PMID: 7832633
-
Glycosylation of the core of the HIV-1 envelope subunit protein gp120 is not required for native trimer formation or viral infectivity.J Biol Chem. 2017 Jun 16;292(24):10197-10219. doi: 10.1074/jbc.M117.788919. Epub 2017 Apr 26. J Biol Chem. 2017. PMID: 28446609 Free PMC article.
-
Glycosylation of HIV-1 gp120 V3 loop: towards the rational design of a synthetic carbohydrate vaccine.Curr Med Chem. 2007;14(30):3232-42. doi: 10.2174/092986707782793826. Curr Med Chem. 2007. PMID: 18220757 Review.
Cited by
-
Predicting the Structures of Glycans, Glycoproteins, and Their Complexes.Chem Rev. 2018 Sep 12;118(17):8005-8024. doi: 10.1021/acs.chemrev.8b00032. Epub 2018 Aug 9. Chem Rev. 2018. PMID: 30091597 Free PMC article. Review.
-
Computational study of HIV gp120 as a target for polyanionic entry inhibitors: Exploiting the V3 loop region.PLoS One. 2018 Jan 18;13(1):e0190658. doi: 10.1371/journal.pone.0190658. eCollection 2018. PLoS One. 2018. PMID: 29346393 Free PMC article.
-
Serine-rich repeat proteins: well-known yet little-understood bacterial adhesins.J Bacteriol. 2024 Jan 25;206(1):e0024123. doi: 10.1128/jb.00241-23. Epub 2023 Nov 17. J Bacteriol. 2024. PMID: 37975670 Free PMC article. Review.
-
Enhanced conformational sampling using replica exchange with concurrent solute scaling and hamiltonian biasing realized in one dimension.J Chem Theory Comput. 2015 Jun 9;11(6):2855-67. doi: 10.1021/acs.jctc.5b00243. J Chem Theory Comput. 2015. PMID: 26082676 Free PMC article.
-
HIV-1 Envelope Conformation, Allostery, and Dynamics.Viruses. 2021 May 7;13(5):852. doi: 10.3390/v13050852. Viruses. 2021. PMID: 34067073 Free PMC article. Review.
References
-
- Wyatt R, Sodroski J (1998) The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280: 1884–1888. - PubMed
-
- Ji X, Chen Y, Faro J, Gewurz H, Bremer J, et al. (2006) Interaction of human immunodeficiency virus (HIV) glycans with lectins of the human immune system. Curr Protein Pept Sci 7: 317–324. - PubMed
-
- Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, et al. (1990) Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem 265: 10373–10382. - PubMed
-
- Myers G, MacInnes K, Korber B (1992) The emergence of simian/human immunodeficiency viruses. AIDS Res Hum Retroviruses 8: 373–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

