Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection
- PMID: 24303043
- PMCID: PMC3841263
- DOI: 10.1371/journal.pone.0081330
Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection
Erratum in
- PLoS One. 2014;9(7):e104471
Abstract
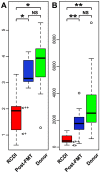
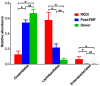
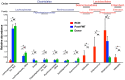
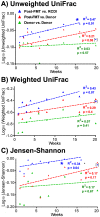
Clostridium difficile causes antibiotic-associated diarrhea and pseudomembraneous colitis and is responsible for a large and increasing fraction of hospital-acquired infections. Fecal microbiota transplantation (FMT) is an alternate treatment option for recurrent C. difficile infection (RCDI) refractory to antibiotic therapy. It has recently been discussed favorably in the clinical and scientific communities and is receiving increasing public attention. However, short- and long-term health consequences of FMT remain a concern, as the effects of the transplanted microbiota on the patient remain unknown. To shed light on microbial events associated with RCDI and treatment by FMT, we performed fecal microbiota analysis by 16S rRNA gene amplicon pyrosequencing of 14 pairs of healthy donors and RCDI patients treated successfully by FMT. Post-FMT patient and healthy donor samples collected up to one year after FMT were studied longitudinally, including one post-FMT patient with antibiotic-associated relapse three months after FMT. This analysis allowed us not only to confirm prior reports that RCDI is associated with reduced diversity and compositional changes in the fecal microbiota, but also to characterize previously undocumented post-FMT microbiota dynamics. Members of the Streptococcaceae, Enterococcaceae, or Enterobacteriaceae were significantly increased and putative butyrate producers, such as Lachnospiraceae and Ruminococcaceae were significantly reduced in samples from RCDI patients before FMT as compared to post-FMT patient and healthy donor samples. RCDI patient samples showed more case-specific variations than post-FMT patient and healthy donor samples. However, none of the bacterial groups were invariably associated with RCDI or successful treatment by FMT. Overall microbiota compositions in post-FMT patients, specifically abundances of the above-mentioned Firmicutes, continued to change for at least 16 weeks after FMT, suggesting that full microbiota recovery from RCDI may take much longer than expected based on the disappearance of diarrheal symptoms immediately after FMT.
Conflict of interest statement
Figures




Similar articles
-
Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection in the Elderly: Long-Term Outcomes and Microbiota Changes.Dig Dis Sci. 2016 Oct;61(10):3007-3015. doi: 10.1007/s10620-016-4229-8. Epub 2016 Jul 22. Dig Dis Sci. 2016. PMID: 27447476
-
Durable Long-Term Bacterial Engraftment following Encapsulated Fecal Microbiota Transplantation To Treat Clostridium difficile Infection.mBio. 2019 Jul 23;10(4):e01586-19. doi: 10.1128/mBio.01586-19. mBio. 2019. PMID: 31337728 Free PMC article.
-
Microbiota transplantation restores normal fecal bile acid composition in recurrent Clostridium difficile infection.Am J Physiol Gastrointest Liver Physiol. 2014 Feb 15;306(4):G310-9. doi: 10.1152/ajpgi.00282.2013. Epub 2013 Nov 27. Am J Physiol Gastrointest Liver Physiol. 2014. PMID: 24284963 Free PMC article.
-
Fecal microbiota transplantation in the treatment of refractory Clostridium difficile infection in children: an update.Curr Opin Pediatr. 2014 Oct;26(5):573-8. doi: 10.1097/MOP.0000000000000127. Curr Opin Pediatr. 2014. PMID: 25046331 Review.
-
Microbiota restoration therapies for recurrent Clostridioides difficile infection reach an important new milestone.Therap Adv Gastroenterol. 2024 May 24;17:17562848241253089. doi: 10.1177/17562848241253089. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 38800353 Free PMC article. Review.
Cited by
-
The Use of Microbiome Restoration Therapeutics to Eliminate Intestinal Colonization With Multidrug-Resistant Organisms.Am J Med Sci. 2018 Nov;356(5):433-440. doi: 10.1016/j.amjms.2018.08.015. Epub 2018 Aug 29. Am J Med Sci. 2018. PMID: 30384952 Free PMC article.
-
The Super-Donor Phenomenon in Fecal Microbiota Transplantation.Front Cell Infect Microbiol. 2019 Jan 21;9:2. doi: 10.3389/fcimb.2019.00002. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 30719428 Free PMC article. Review.
-
Safety and feasibility of faecal microbiota transplantation for patients with Parkinson's disease: a protocol for a self-controlled interventional donor-FMT pilot study.BMJ Open. 2023 Oct 5;13(10):e071766. doi: 10.1136/bmjopen-2023-071766. BMJ Open. 2023. PMID: 37798034 Free PMC article. Clinical Trial.
-
One dog's waste is another dog's wealth: A pilot study of fecal microbiota transplantation in dogs with acute hemorrhagic diarrhea syndrome.PLoS One. 2021 Apr 19;16(4):e0250344. doi: 10.1371/journal.pone.0250344. eCollection 2021. PLoS One. 2021. PMID: 33872339 Free PMC article.
-
GUTSS: An Alignment-Free Sequence Comparison Method for Use in Human Intestinal Microbiome and Fecal Microbiota Transplantation Analysis.PLoS One. 2016 Jul 8;11(7):e0158897. doi: 10.1371/journal.pone.0158897. eCollection 2016. PLoS One. 2016. PMID: 27391011 Free PMC article. Clinical Trial.
References
-
- Bartlett JG (2002) Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 346: 334–339. - PubMed
-
- Jobe BA, Grasley A, Deveney KE, Deveney CW, Sheppard BC (1995) Clostridium difficile colitis: an increasing hospital-acquired illness. Am J Surg 169: 480–483. - PubMed
-
- Miller BA, Chen LF, Sexton DJ, Anderson DJ (2011) Comparison of the burdens of hospital-onset, healthcare facility-associated Clostridium difficile Infection and of healthcare-associated infection due to methicillin-resistant Staphylococcus aureus in community hospitals. Infect Control Hosp Epidemiol 32: 387–390. - PubMed
-
- Rivera EV, Woods S (2003) Prevalence of asymptomatic Clostridium difficile colonization in a nursing home population: a cross-sectional study. J Gend Specif Med 6: 27–30. - PubMed
-
- Jangi S, Lamont JT (2010) Asymptomatic colonization by Clostridium difficile in infants: implications for disease in later life. J Pediatr Gastroenterol Nutr 51: 2–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

