WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation
- PMID: 24303070
- PMCID: PMC3841149
- DOI: 10.1371/journal.pone.0081769
WNT3 inhibits cerebellar granule neuron progenitor proliferation and medulloblastoma formation via MAPK activation
Abstract
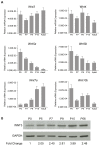
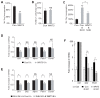
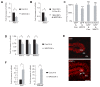
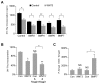
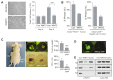
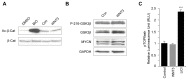
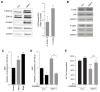
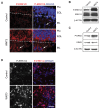
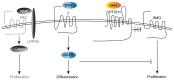
During normal cerebellar development, the remarkable expansion of granule cell progenitors (GCPs) generates a population of granule neurons that outnumbers the total neuronal population of the cerebral cortex, and provides a model for identifying signaling pathways that may be defective in medulloblastoma. While many studies focus on identifying pathways that promote growth of GCPs, a critical unanswered question concerns the identification of signaling pathways that block mitogenic stimulation and induce early steps in differentiation. Here we identify WNT3 as a novel suppressor of GCP proliferation during cerebellar development and an inhibitor of medulloblastoma growth in mice. WNT3, produced in early postnatal cerebellum, inhibits GCP proliferation by down-regulating pro-proliferative target genes of the mitogen Sonic Hedgehog (SHH) and the bHLH transcription factor Atoh1. WNT3 suppresses GCP growth through a non-canonical Wnt signaling pathway, activating prototypic mitogen-activated protein kinases (MAPKs), the Ras-dependent extracellular-signal-regulated kinases 1/2 (ERK1/2) and ERK5, instead of the classical β-catenin pathway. Inhibition of MAPK activity using a MAPK kinase (MEK) inhibitor reversed the inhibitory effect of WNT3 on GCP proliferation. Importantly, WNT3 inhibits proliferation of medulloblastoma tumor growth in mouse models by a similar mechanism. Thus, the present study suggests a novel role for WNT3 as a regulator of neurogenesis and repressor of neural tumors.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

