Animal model of simulated microgravity: a comparative study of hindlimb unloading via tail versus pelvic suspension
- PMID: 24303103
- PMCID: PMC3831940
- DOI: 10.1002/phy2.12
Animal model of simulated microgravity: a comparative study of hindlimb unloading via tail versus pelvic suspension
Abstract
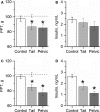
The aim of this study was to compare physiological effects of hindlimb suspension (HLS) in tail- and pelvic-HLS rat models to determine if severe stretch in the tail-HLS rats lumbosacral skeleton may contribute to the changes traditionally attributed to simulated microgravity and musculoskeletal disuse in the tail-HLS model. Adult male Sprague-Dawley rats divided into suspended and control-nonsuspended groups were subjected to two separate methods of suspension and maintained with regular food and water for 2 weeks. Body weights, food and water consumption, soleus muscle weight, tibial bone mineral density, random plasma insulin, and hindlimb pain on pressure threshold (PPT) were measured. X-ray analysis demonstrated severe lordosis in tail- but not pelvic-HLS animals. However, growth retardation, food consumption, and soleus muscle weight and tibial bone density (decreased relative to control) did not differ between two HLS models. Furthermore, HLS rats developed similar levels of insulinopenia and mechanical hyperalgesia (decreased PPT) in both tail- and pelvic-HLS groups. In the rat-to-rat comparisons, the growth retardation and the decreased PPT observed in HLS-rats was most associated with insulinopenia. In conclusion, these data suggest that HLS results in mild prediabetic state with some signs of pressure hyperalgesia, but lumbosacral skeleton stretch plays little role, if any, in these pathological changes.
Keywords: Hindlimb unloading; insulin; neuropathy; prediabetes; pressure hyperalgesia.
Figures





References
-
- Allen MR, Hogan HA, Bloomfield SA. Differential bone and muscle recovery following hindlimb unloading in skeletally mature male rats. J. Musculoskelet. Neuronal Interact. 2006;6:217–225. - PubMed
-
- Andersson G. Burden of musculoskeletal diseases in the United States: prevalence, societal and economic cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008.
-
- Benedetti M, Merino R, Kusuda R, Ravanelli MI, Cadetti F, dos Santos P, et al. Plasma corticosterone levels in mouse models of pain. Eur. J. Pain. 2012;16:803–815. - PubMed
-
- Bloomfield SA, Allen MR, Hogan HA, Delp MD. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone. 2002;31:149–157. - PubMed
-
- Carpenter RD, Lang TF, Bloomfield SA, Bloomberg JJ, Judex S, Keyak JH, et al. Effects of long-duration spaceflight, microgravity, and radiation on the neuromuscular, sensorimotor, and skeletal systems. J. Cosmol. 2010;12:3778–3780.
LinkOut - more resources
Full Text Sources
Other Literature Sources

