Stabilization of the Central Part of Tropomyosin Molecule Alters the Ca2+-sensitivity of Actin-Myosin Interaction
- PMID: 24303208
- PMCID: PMC3848074
Stabilization of the Central Part of Tropomyosin Molecule Alters the Ca2+-sensitivity of Actin-Myosin Interaction
Abstract
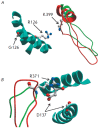
We show that the mutations D137L and G126R, which stabilize the central part of the tropomyosin (Tm) molecule, increase both the maximal sliding velocity of the regulated actin filaments in the in vitro motility assay at high Са(2+) concentrations and the Са(2+)-sensitivity of the actin-myosin interaction underlying this sliding. Based on an analysis of the recently published data on the structure of the actin-Tm-myosin complex, we suppose that the physiological effects of these mutations in Tm can be accounted for by their influence on the interactions between the central part of Tm and certain sites of the myosin head.
Keywords: actin-myosin interaction; in vitro motility assay; regulation of muscle contraction; tropomyosin.
Figures

References
-
- Nevzorov I.A., Levitsky D.I.. Biochemistry (Moscow). 2077;76(13):1507–1527. - PubMed
-
- Sumida J.P., Wu E., Lehrer S.S.. J. Biol. Chem. 2008;283:6728–6734. - PubMed
-
- Shchepkin D.V., Kopylova G.V., Nikitina L.V., Katsnelson L.B., Bershitsky S.Y.. Biochem. Biophys. Res. Commun. 2010;401(1):159–163. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
