Automated Detection of Systematic Off-label Drug Use in Free Text of Electronic Medical Records
- PMID: 24303308
- PMCID: PMC3814472
Automated Detection of Systematic Off-label Drug Use in Free Text of Electronic Medical Records
Abstract
Off-label use of a drug occurs when it is used in a manner that deviates from its FDA label. Studies estimate that 21% of prescriptions are off-label, with only 27% of those uses supported by evidence of safety and efficacy. We have developed methods to detect population level off-label usage using computationally efficient annotation of free text from clinical notes to generate features encoding empirical information about drug-disease mentions. By including additional features encoding prior knowledge about drugs, diseases, and known usage, we trained a highly accurate predictive model that was used to detect novel candidate off-label usages in a very large clinical corpus. We show that the candidate uses are plausible and can be prioritized for further analysis in terms of safety and efficacy.
Figures
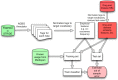

References
-
- Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Archives of internal medicine. 2006;166(9):1021–6. Epub 2006/05/10. - PubMed
-
- Chen DT, Wynia MK, Moloney RM, Alexander GC. U.S. physician knowledge of the FDA-approved indications and evidence base for commonly prescribed drugs: results of a national survey. Pharmacoepidemiology and drug safety. 2009;18(11):1094–100. Epub 2009/08/22. - PubMed
-
- Dal Pan GJ. Monitoring the safety of medicines used off-label. Clinical pharmacology and therapeutics. 2012;91(5):787–95. Epub 2012/04/05. - PubMed
-
- Flowers CM, Racoosin JA, Kortepeter C. Seizure activity and off-label use of tiagabine. The New England journal of medicine. 2006;354(7):773–4. Epub 2006/02/17. - PubMed
