Intratracheal exposure to Fab fragments of an allergen-specific monoclonal antibody regulates asthmatic responses in mice
- PMID: 24303921
- PMCID: PMC3956435
- DOI: 10.1111/imm.12225
Intratracheal exposure to Fab fragments of an allergen-specific monoclonal antibody regulates asthmatic responses in mice
Abstract
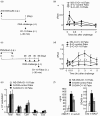
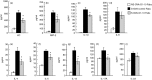
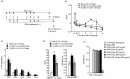
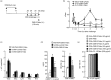
Fab fragments (Fabs) maintain the ability to bind to specific antigens but lack effector functions due to the absence of the Fc portion. In the present study, we tested whether Fabs of an allergen-specific monoclonal antibody (mAb) were able to regulate asthmatic responses in mice. Asthmatic responses were induced in BALB/c mice by passive sensitization with anti-ovalbumin (OVA) polyclonal antibodies (pAbs) (day 0) and by active sensitization with OVA (days 0 and 14), followed by intratracheal (i.t.) challenge with OVA on day 1 and days 28, 29, 30 and 35. Fabs prepared by the digestion of an anti-OVA IgG1 (O1-10) mAb with papain were i.t. administered only once 30 min before antigenic challenge on day 1 or day 35. The results showed that i.t. administration of O1-10 Fabs with OVA markedly suppressed the early and/or late phases of asthmatic responses caused by passive and active sensitization. Similar results were obtained when Fabs of anti-OVA IgG2b mAb (O2B-3) were i.t. administered. In contrast, neither i.t. injection of intact 01-10/O2B-3 nor systemic injection of O1-10 Fabs suppressed the asthmatic responses. In vitro studies revealed that the capture of OVA by O1-10 Fabs prevented the subsequent binding of intact anti-OVA pAbs to the captured OVA. These results suggest that asthmatic responses may be down-regulated by the i.t. exposure to Fabs of an allergen-specific mAb via a mechanism involving the capture of allergen by Fabs in the respiratory tract before the interaction of intact antibody and allergen essential for the induction of asthmatic responses.
Keywords: Fab; asthma; intratracheal exposure; monoclonal antibody.
© 2013 John Wiley & Sons Ltd.
Figures

References
-
- Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. - PubMed
-
- Robinson DS, Hamid Q, Ying S, et al. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. - PubMed
-
- Fireman P. Understanding asthma pathophysiology. Allergy Asthma Proc. 2003;24:79–83. - PubMed
-
- Cockroft DW. Allergen-induced increase in nonallergic airway responsiveness; a citation classic revisited. Can Respir J. 2000;7:182–7. - PubMed
-
- Durham SR. The significance of late responses in asthma. Clin Exp Allergy. 1991;21:3–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

