Asbestos-associated mesothelial cell autoantibodies promote collagen deposition in vitro
- PMID: 24304304
- PMCID: PMC5002354
- DOI: 10.3109/08958378.2013.848249
Asbestos-associated mesothelial cell autoantibodies promote collagen deposition in vitro
Abstract
Fibrosis, characterized by excessive collagen protein deposition, is a progressive disease that can fatally inhibit organ function. Prolonged exposure to pathogens or environmental toxicants such as asbestos can lead to chronic inflammatory responses associated with fibrosis. Significant exposure to amphibole asbestos has been reported in and around Libby, Montana due to local mining of asbestos-contaminated vermiculite. These exposures have been implicated in a unique disease etiology characterized predominantly by pleural disorders, including fibrosis. We recently reported the discovery of mesothelial cell autoantibodies (MCAAs) in the sera of Libby residents and demonstrated a positive and significant correlation with pleural disease; however, a mechanistic link was not determined. Here we demonstrate that MCAAs induce pleural mesothelial cells to produce a collagen matrix but do not affect production of the pro-inflammatory cytokine tumor growth factor-β. While autoantibodies commonly induce a pro-fibrotic state by inducing epithelial-mesenchymal transition (EMT) of target cells, we found no evidence supporting EMT in cells exposed to MCAA positive human sera. Although implicated in other models of pulmonary fibrosis, activity of the protein SPARC (secreted protein, acidic and rich in cysteine) did not affect MCAA-induced collagen deposition. However, matrix formation was dependent on matrix metalloproteinase (MMP) activity, and we noted increased expression of MMP-8 and -9 in supernatants of mesothelial cells incubated with MCAA positive sera compared to control. These data suggest a mechanism by which MCAA binding leads to increased collagen deposition through altering MMP expression and provides an important mechanistic link between MCAAs and asbestos-related, autoimmune-induced pleural fibrosis.
Conflict of interest statement
Declaration of interest All the authors report no declaration of interest. This work was supported by CDC/ATSDR R01 Grant TS000099-01, the Libby Epidemiology Research Program (LERP) and Idaho State University’s University Research Council Graduate Student grant to K.M.S. Core facilities that supported this work were funded in part by NIH grant P20 RR016454 (INBRE). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Toxic Substances and Disease Registry.
Figures
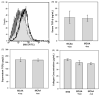
 ) induced SMA expression compared with exposure to secondary antibody control (
) induced SMA expression compared with exposure to secondary antibody control (
 ,
,
 ), MCAA +ive (
), MCAA +ive (
 ,
,
 ), or MCAA −ive (
), or MCAA −ive (
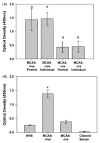
 ) sera. Exposure to MCAA +ive or −ive sera resulted in increased SMA expression over secondary control (x-axis) but expression did not increase to the same degree as TGF-β exposure. TGF-β was detected in MCAA +ive and −ive sera (B) and supernatants (C) of cells exposed to these sera samples. TGF-β was detected using a cytokine bead array analysis and concentrations suggest that the TGF-β detected in supernatants was added upon addition of sera and not produced by the mesothelial cells, mean ± SD, n =3). (D) Total soluble collagen concentrations in cell supernatants were determined using a Sircol Soluble Collagen Assay. Incubation with sera from asbestos-exposed subjects did not significantly affect soluble collagen compared to NHS.
) sera. Exposure to MCAA +ive or −ive sera resulted in increased SMA expression over secondary control (x-axis) but expression did not increase to the same degree as TGF-β exposure. TGF-β was detected in MCAA +ive and −ive sera (B) and supernatants (C) of cells exposed to these sera samples. TGF-β was detected using a cytokine bead array analysis and concentrations suggest that the TGF-β detected in supernatants was added upon addition of sera and not produced by the mesothelial cells, mean ± SD, n =3). (D) Total soluble collagen concentrations in cell supernatants were determined using a Sircol Soluble Collagen Assay. Incubation with sera from asbestos-exposed subjects did not significantly affect soluble collagen compared to NHS.
 ) did not significantly alter detection of collagen proteins compared to no treatment (■), indicating that SPARC does not directly affect MCAA-induced collagen deposition, as determined by two-way ANOVA, mean ±SE, n =4.
) did not significantly alter detection of collagen proteins compared to no treatment (■), indicating that SPARC does not directly affect MCAA-induced collagen deposition, as determined by two-way ANOVA, mean ±SE, n =4.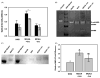
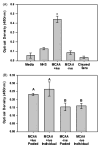
 ) of the non-specific MMP inhibitor EDTA (5 mM). Collagen deposition was significantly inhibited by EDTA in cells exposed to MCAA +ive sera (*p =0.018) but not to NHS (p =0.28) or MCAA −ive sera (p =0.32) as determined by two-way ANOVA. (B) Collagen zymography revealed the presence of MMP-8 and MMP-9 in supernatants of cells exposed to human sera for 4 days. MMPs were identified by their reported molecular weights and bacterial collagenase (200 pg) was used as a positive control. (C) Immunoblot analysis of MMP-8 confirmed its identity. (D) Densitometry analysis of the immunoblot revealed significantly more MMP-8 present in supernatants of cells exposed to MCAA +ive sera compared to NHS. However, exposure to MCAA −ive sera also resulted in increased MMP-8, although this increase was not significant compared to NHS. Statistical significance is indicated by different letters at p<0.01 by one-way ANOVA, mean ±SD, n =3.
) of the non-specific MMP inhibitor EDTA (5 mM). Collagen deposition was significantly inhibited by EDTA in cells exposed to MCAA +ive sera (*p =0.018) but not to NHS (p =0.28) or MCAA −ive sera (p =0.32) as determined by two-way ANOVA. (B) Collagen zymography revealed the presence of MMP-8 and MMP-9 in supernatants of cells exposed to human sera for 4 days. MMPs were identified by their reported molecular weights and bacterial collagenase (200 pg) was used as a positive control. (C) Immunoblot analysis of MMP-8 confirmed its identity. (D) Densitometry analysis of the immunoblot revealed significantly more MMP-8 present in supernatants of cells exposed to MCAA +ive sera compared to NHS. However, exposure to MCAA −ive sera also resulted in increased MMP-8, although this increase was not significant compared to NHS. Statistical significance is indicated by different letters at p<0.01 by one-way ANOVA, mean ±SD, n =3.Similar articles
-
Libby amphibole-induced mesothelial cell autoantibodies bind to surface plasminogen and alter collagen matrix remodeling.Physiol Rep. 2016 Aug;4(15):e12881. doi: 10.14814/phy2.12881. Physiol Rep. 2016. PMID: 27519611 Free PMC article.
-
Mesothelial cell autoantibodies upregulate transcription factors associated with fibrosis.Inhal Toxicol. 2017 Jan;29(1):10-17. doi: 10.1080/08958378.2016.1271841. Inhal Toxicol. 2017. PMID: 28183202 Free PMC article.
-
Libby amphibole-induced mesothelial cell autoantibodies promote collagen deposition in mice.Am J Physiol Lung Cell Mol Physiol. 2016 Jun 1;310(11):L1071-7. doi: 10.1152/ajplung.00462.2015. Epub 2016 Apr 22. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27106292 Free PMC article.
-
Amphibole asbestos in tree bark--a review of findings for this inhalational exposure source in Libby, Montana.J Occup Environ Hyg. 2012;9(6):387-97. doi: 10.1080/15459624.2012.682217. J Occup Environ Hyg. 2012. PMID: 22577793 Review.
-
A review of scientific literature examining the mining history, geology, mineralogy, and amphibole asbestos health effects of the Rainy Creek igneous complex, Libby, Montana, USA.Inhal Toxicol. 2006 Nov;18(12):949-62. doi: 10.1080/08958370600834982. Inhal Toxicol. 2006. PMID: 16920668 Review.
Cited by
-
Plasminogen Binding and Activation at the Mesothelial Cell Surface Promotes Invasion through a Collagen Matrix.Int J Mol Sci. 2022 May 26;23(11):5984. doi: 10.3390/ijms23115984. Int J Mol Sci. 2022. PMID: 35682663 Free PMC article.
-
Libby amphibole-induced mesothelial cell autoantibodies bind to surface plasminogen and alter collagen matrix remodeling.Physiol Rep. 2016 Aug;4(15):e12881. doi: 10.14814/phy2.12881. Physiol Rep. 2016. PMID: 27519611 Free PMC article.
-
Mesothelial cell autoantibodies upregulate transcription factors associated with fibrosis.Inhal Toxicol. 2017 Jan;29(1):10-17. doi: 10.1080/08958378.2016.1271841. Inhal Toxicol. 2017. PMID: 28183202 Free PMC article.
-
Libby amphibole-induced mesothelial cell autoantibodies promote collagen deposition in mice.Am J Physiol Lung Cell Mol Physiol. 2016 Jun 1;310(11):L1071-7. doi: 10.1152/ajplung.00462.2015. Epub 2016 Apr 22. Am J Physiol Lung Cell Mol Physiol. 2016. PMID: 27106292 Free PMC article.
-
Autoimmune markers for progression of Libby amphibole lamellar pleural thickening.Inhal Toxicol. 2019 Sep-Oct;31(11-12):409-419. doi: 10.1080/08958378.2019.1699616. Epub 2019 Dec 8. Inhal Toxicol. 2019. PMID: 31814459 Free PMC article.
References
-
- Antony VB, Hott JW, Kunkel SL, et al. Pleural mesothelial cell expression of C-C (monocyte chemotactic peptide) and C-X-C (interleukin 8) chemokines. Am J Respir Cell Mol Biol. 1995;12:581–8. - PubMed
-
- Bandli BR, Gunter ME. A review of scientific literature examining the mining history, geology, mineralogy, and amphibole asbestos health effects of the Rainy Creek igneous complex, Libby, Montana, USA. Inhal Toxicol. 2006;18:949–62. - PubMed
-
- Baroni SS, Santillo M, Bevilacqua F, et al. Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. N Engl J Med. 2006;354:2667–76. - PubMed
-
- Chizzolini C, Raschi E, Rezzonico R, et al. Autoantibodies to fibroblasts induce a proadhesive and proinflammatory fibroblast phenotype in patients with systemic sclerosis. Arthritis Rheum. 2002;46:1602–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
