Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy
- PMID: 24304438
- PMCID: PMC4498907
- DOI: 10.1111/epi.12434
Electroencephalography/functional magnetic resonance imaging responses help predict surgical outcome in focal epilepsy
Abstract
Purpose: Simultaneous electroencephalography/functional magnetic resonance imaging (EEG/fMRI) recording can noninvasively map in the whole brain the hemodynamic response following an interictal epileptic discharge. EEG/fMRI is gaining interest as a presurgical evaluation tool. This study aims to determine how hemodynamic responses related to epileptic activity can help predict surgical outcome in patients considered for epilepsy surgery.
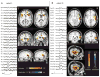
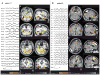
Methods: Thirty-five consecutive patients with focal epilepsy who had significant hemodynamic responses and eventually surgical resection, were studied. The statistical map of hemodynamic responses were generated and co-registered to postoperative anatomic imaging. Patients were classified into four groups defined by the relative relationship between the location of the maximum hemodynamic response and the resection: group 1, fully concordant; group 2, partially concordant; group 3, partially discordant; and group 4, fully discordant. These findings were correlated with surgical outcome with at least 12-month follow-up.
Key findings: Ten patients in group 1 had the maximum t value (t-max) inside the resection; nine in group 2 had the t-max outside but close to the resection and the cluster with t-max overlapped the resection; five in group 3 had the t-max remote from resection, but with another less significant cluster in the resection; and 11 in group 4 had no response in the resection. The degree of concordance correlated largely with surgical outcome: a good surgical outcome (Engel's class I) was found in 7 of 10 patients of group 1, 4 of 9 of group 2, 3 of 5 of group 3, and only 1 of 11 of group 4. These results indicate that the partially concordant and partially discordant groups are best considered as inconclusive. In contrast, in the fully concordant and fully discordant groups, the sensitivity, specificity, positive predictive value, and negative predictive value were high, 87.5%, 76.9%, 70%, and 90.9%, respectively.
Significance: This study demonstrates that hemodynamic responses related to epileptic activity can help delineate the epileptogenic region. Full concordance between maximum response and surgical resection is indicative of seizure freedom, whereas a resection leaving the maximum response intact is likely to lead to a poor outcome. EEG/fMRI is noninvasive but is limited to patients in whom interictal epileptic discharges can be recorded during the 60-90 min scan.
Keywords: Electroencephalography/functional magnetic resonance imaging; Epilepsy surgery; Outcome.
Wiley Periodicals, Inc. © 2013 International League Against Epilepsy.
Figures



References
-
- Schuele SU, Luders HO. Intractable epilepsy: management and therapeutic alternatives. Lancet Neurol. 2008;7:514–524. - PubMed
-
- Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. - PubMed
-
- Engel J., Jr Surgery for seizures. N Engl J Med. 1996;334:647–652. - PubMed
-
- Laufs H, Duncan JS. Electroencephalography/functional MRI in human epilepsy: what it currently can and cannot do. Curr Opin Neurol. 2007;20:417–423. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

