Synchronized firing of fast-spiking interneurons is critical to maintain balanced firing between direct and indirect pathway neurons of the striatum
- PMID: 24304860
- PMCID: PMC3921391
- DOI: 10.1152/jn.00382.2013
Synchronized firing of fast-spiking interneurons is critical to maintain balanced firing between direct and indirect pathway neurons of the striatum
Abstract
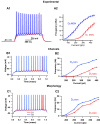
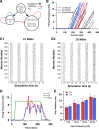
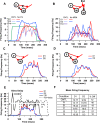
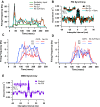
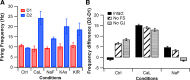
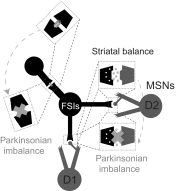
The inhibitory circuits of the striatum are known to be critical for motor function, yet their contributions to Parkinsonian motor deficits are not clear. Altered firing in the globus pallidus suggests that striatal medium spiny neurons (MSN) of the direct (D1 MSN) and indirect pathway (D2 MSN) are imbalanced during dopamine depletion. Both MSN classes receive inhibitory input from each other and from inhibitory interneurons within the striatum, specifically the fast-spiking interneurons (FSI). To investigate the role of inhibition in maintaining striatal balance, we developed a biologically-realistic striatal network model consisting of multicompartmental neuron models: 500 D1 MSNs, 500 D2 MSNs and 49 FSIs. The D1 and D2 MSN models are differentiated based on published experiments of individual channel modulations by dopamine, with D2 MSNs being more excitable than D1 MSNs. Despite this difference in response to current injection, in the network D1 and D2 MSNs fire at similar frequencies in response to excitatory synaptic input. Simulations further reveal that inhibition from FSIs connected by gap junctions is critical to produce balanced firing. Although gap junctions produce only a small increase in synchronization between FSIs, removing these connections resulted in significant firing differences between D1 and D2 MSNs, and balanced firing was restored by providing synchronized cortical input to the FSIs. Together these findings suggest that desynchronization of FSI firing is sufficient to alter balanced firing between D1 and D2 MSNs.
Keywords: Parkinson's disease; fast-spiking interneuron; gap junctions; medium-spiny neuron; striatum.
Figures
Similar articles
-
Desynchronization of fast-spiking interneurons reduces β-band oscillations and imbalance in firing in the dopamine-depleted striatum.J Neurosci. 2015 Jan 21;35(3):1149-59. doi: 10.1523/JNEUROSCI.3490-14.2015. J Neurosci. 2015. PMID: 25609629 Free PMC article.
-
Dopaminergic treatment weakens medium spiny neuron collateral inhibition in the parkinsonian striatum.J Neurophysiol. 2017 Mar 1;117(3):987-999. doi: 10.1152/jn.00683.2016. Epub 2016 Dec 7. J Neurophysiol. 2017. PMID: 27927785 Free PMC article.
-
Representation of the body in the lateral striatum of the freely moving rat: Fast Spiking Interneurons respond to stimulation of individual body parts.Brain Res. 2017 Feb 15;1657:101-108. doi: 10.1016/j.brainres.2016.11.033. Epub 2016 Nov 30. Brain Res. 2017. PMID: 27914882 Free PMC article.
-
Modeling influences of dopamine on synchronization behavior of striatum.Network. 2017;28(1):28-52. doi: 10.1080/0954898X.2017.1378824. Epub 2017 Oct 6. Network. 2017. PMID: 28985088 Review.
-
Optogenetic insights into striatal function and behavior.Behav Brain Res. 2013 Oct 15;255:44-54. doi: 10.1016/j.bbr.2013.04.018. Epub 2013 Apr 28. Behav Brain Res. 2013. PMID: 23628212 Review.
Cited by
-
Npas1+ Pallidal Neurons Target Striatal Projection Neurons.J Neurosci. 2016 May 18;36(20):5472-88. doi: 10.1523/JNEUROSCI.1720-15.2016. J Neurosci. 2016. PMID: 27194328 Free PMC article.
-
Cortical control of striatal fast-spiking interneuron synchrony.J Physiol. 2022 May;600(9):2189-2202. doi: 10.1113/JP282850. Epub 2022 Apr 11. J Physiol. 2022. PMID: 35332539 Free PMC article.
-
Parvalbumin+ Neurons and Npas1+ Neurons Are Distinct Neuron Classes in the Mouse External Globus Pallidus.J Neurosci. 2015 Aug 26;35(34):11830-47. doi: 10.1523/JNEUROSCI.4672-14.2015. J Neurosci. 2015. PMID: 26311767 Free PMC article.
-
Existence and control of Go/No-Go decision transition threshold in the striatum.PLoS Comput Biol. 2015 Apr 24;11(4):e1004233. doi: 10.1371/journal.pcbi.1004233. eCollection 2015 Apr. PLoS Comput Biol. 2015. PMID: 25910230 Free PMC article.
-
Pallidal gap junctions-triggers of synchrony in Parkinson's disease?Mov Disord. 2014 Oct;29(12):1486-94. doi: 10.1002/mds.25987. Epub 2014 Aug 13. Mov Disord. 2014. PMID: 25124148 Free PMC article.
References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 - PubMed
-
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience 62: 707–719, 1994 - PubMed
-
- Berke JD, Okatan M, Skurski J, Eichenbaum HB. Oscillatory entrainment of striatal neurons in freely moving rats. Neuron 43: 883–896, 2004 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

