A novel approach to rapidly prevent age-related cognitive decline
- PMID: 24305557
- PMCID: PMC4331782
- DOI: 10.1111/acel.12178
A novel approach to rapidly prevent age-related cognitive decline
Abstract
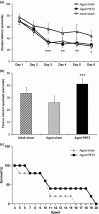
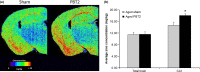
The loss of cognitive function is a pervasive and often debilitating feature of the aging process for which there are no effective therapeutics. We hypothesized that a novel metal chaperone (PBT2; Prana Biotechnology, Parkville, Victoria, Australia) would enhance cognition in aged rodents. We show here that PBT2 rapidly improves the performance of aged C57Bl/6 mice in the Morris water maze, concomitant with increases in dendritic spine density, hippocampal neuron number and markers of neurogenesis. There were also increased levels of specific glutamate receptors (alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid and N-methyl-D-aspartate), the glutamate transporter (VGLUT1) and glutamate itself. Markers of synaptic plasticity [calmodulin-dependent protein kinase II (CaMKII) and phosphorylated CaMKII, CREB, synaptophysin] were also increased following PBT2 treatment. We also demonstrate that PBT2 treatment results in a subregion-specific increase in hippocampal zinc, which is increasingly recognized as a potent neuromodulator. These data demonstrate that metal chaperones are a novel approach to the treatment of age-related cognitive decline.
Keywords: PBT2; aging; anti-aging; cognition; zinc.
© 2013 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures


References
-
- Adlard PA, Cherny RA, Finkelstein DI, Gautier E, Robb E, Cortes M, Volitakis I, Liu X, Smith JP, Perez K, Laughton K, Li QX, Charman SA, Nicolazzo JA, Wilkins S, Deleva K, Lynch T, Kok G, Ritchie CW, Tanzi RE, Cappai R, Masters CL, Barnham KJ, Bush AI. Rapid restoration of cognition in Alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial Abeta. Neuron. 2008;59:43–55. - PubMed
-
- Albert M, Duffy FH, Naeser M. Nonlinear changes in cognition with age and their neuropsychologic correlates. Can. J. Psychol. 1987;41:141–157. - PubMed
-
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl Acad. Sci. USA. 1999;96:5280–5285. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

