Embryonic expression of the common progeroid lamin A splice mutation arrests postnatal skin development
- PMID: 24305605
- PMCID: PMC4331787
- DOI: 10.1111/acel.12173
Embryonic expression of the common progeroid lamin A splice mutation arrests postnatal skin development
Abstract
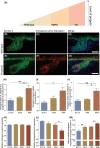
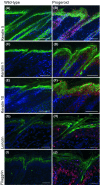
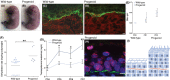
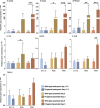
Hutchinson-Gilford progeria syndrome (HGPS) and restrictive dermopathy (RD) are two laminopathies caused by mutations leading to cellular accumulation of prelamin A or one of its truncated forms, progerin. One proposed mechanism for the more severe symptoms in patients with RD compared with HGPS is that higher levels of farnesylated lamin A are produced in RD. Here, we show evidence in support of that hypothesis. Overexpression of the most common progeroid lamin A mutation (LMNA c.1824C>T, p.G608G) during skin development results in a severe phenotype, characterized by dry scaly skin. At postnatal day 5 (PD5), progeroid animals showed a hyperplastic epidermis, disorganized sebaceous glands and an acute inflammatory dermal response, also involving the hypodermal fat layer. PD5 animals also showed an upregulation of multiple inflammatory response genes and an activated NF-kB target pathway. Careful analysis of the interfollicular epidermis showed aberrant expression of the lamin B receptor (LBR) in the suprabasal layer. Prolonged expression of LBR, in 14.06% of the cells, likely contributes to the observed arrest of skin development, clearly evident at PD4 when the skin had developed into single-layer epithelium in the wild-type animals while progeroid animals still had the multilayered appearance typical for skin at PD3. Suprabasal cells expressing LBR showed altered DNA distribution, suggesting the induction of gene expression changes. Despite the formation of a functional epidermal barrier and proven functionality of the gap junctions, progeroid animals displayed a greater rate of water loss as compared with wild-type littermates and died within the first two postnatal weeks.
Keywords: HGPS; aging; lamin B; lamin B receptor; progerin; restrictive dermopathy.
© 2014 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures

References
-
- Capell B, Collins F. Human laminopathies: nuclei gone genetically awry. Nat. Rev. Genet. 2006;7:940–952. - PubMed
-
- Charles R-P, Guitard M, Leyvraz C, Breiden B, Haftek M, Haftek-Terreau Z, Stehle J-C, Sandhoff K, Hummler E. Postnatal requirement of the epithelial sodium channel for maintenance of epidermal barrier function. J. Biol. Chem. 2008;283:2622–2630. - PubMed
-
- Dean JC, Gray ES, Stewart KN, Brown T, Lloyd DJ, Smith NC, Pope FM. Restrictive dermopathy: a disorder of skin differentiation with abnormal integrin expression. Clin. Genet. 1993;44:287–291. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

