MiR-328 promotes glioma cell invasion via SFRP1-dependent Wnt-signaling activation
- PMID: 24305703
- PMCID: PMC3895379
- DOI: 10.1093/neuonc/not164
MiR-328 promotes glioma cell invasion via SFRP1-dependent Wnt-signaling activation
Abstract
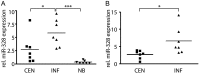
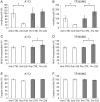
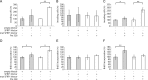
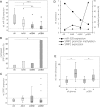
Background Diffusely infiltrative growth of human astrocytic gliomas is one of the major obstacles to successful tumor therapy. Thorough insights into the molecules and pathways signaling glioma cell invasion thus appear of major relevance for the development of targeted and individualized therapies. By miRNA expression profiling of microdissected human tumor biopsy specimens we identified miR-328 as one of the main miRNAs upregulated in invading glioma cells in vivo and further investigated its role in glioma pathogenesis. Methods We employed miRNA mimics and inhibitors to functionally characterize miR-328, 3' untranslated region luciferase assays, and T-cell factor/lymphoid enhancer factor reporter assays to pinpoint miR-328 targets and signaling pathways, and analyzed miR-328 expression in a large panel of gliomas. Results First, we corroborated the invasion-promoting role of miR-328 in A172 and TP365MG glioma cells. Secreted Frizzled-related protein 1 (SFRP1), an inhibitor of Wnt signaling, was then pinpointed as a direct miR-328 target. SFRP1 expression is of prognostic relevance in gliomas with reduced expression, being associated with significantly lower overall patient survival in both the Repository of Molecular Brain Neoplasia Data (REMBRANDT) and The Cancer Genome Atlas. Of note, miR-328 regulated both SFRP1 protein expression levels and Wnt signaling pathway activity. Finally, in human glioma tissues miR-328 appeared to account for the downregulation of SFRP1 preferentially in lower-grade astrocytic gliomas and was inversely related to SFRP1 promoter hypermethylation. Conclusion Taken together, we report on a novel molecular miR-328-dependent mechanism that via SFRP1 inhibition and Wnt activation contributes to the infiltrative glioma phenotype at already early stages of glioma progression, with unfavorable prognostic implications for the final outcome of the disease.
Keywords: astrocytoma; brain tumor; epigenetic; glioblastoma; miRNA.
Figures



References
-
- Kamino M, Kishida M, Kibe T, et al. Wnt-5a signaling is correlated with infiltrative activity in human glioma by inducing cellular migration and MMP-2. Cancer Sci. 2011;102(3):540–548. - PubMed
-
- Roth W, Wild-Bode C, Platten M, et al. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19(37):4210–4220. - PubMed
-
- Götze S, Wolter M, Reifenberger G, Muller O, Sievers S. Frequent promoter hypermethylation of Wnt pathway inhibitor genes in malignant astrocytic gliomas. Int J Cancer. 2010;126(11):2584–2593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

