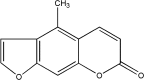
Bergapten prevents lipopolysaccharide mediated osteoclast formation, bone resorption and osteoclast survival
- PMID: 24305787
- PMCID: PMC3936084
- DOI: 10.1007/s00264-013-2184-y
Bergapten prevents lipopolysaccharide mediated osteoclast formation, bone resorption and osteoclast survival
Abstract
Purpose: This study was designed to investigate the potential effect of bergapten on lipopolysaccharide (LPS)-mediated osteoclast formation, bone resorption and osteoclast survival in vitro.
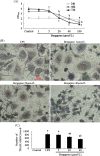
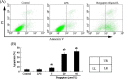
Methods: After osteoclast precursor RAW264.7 cells were treated with bergapten (5, 20, 40 μmol/L) for 72 hours in the presence of LPS (100 ng/ml), osteoclastogenesis was identified by tartrate-resistant acid phosphatase (TRAP) staining, and the number of TRAP-positive multinucleated cells [TRAP(+)MNCs] per well were counted. To investigate the effect of bergapten on osteoclastic bone resorption, RAW264.7 cells were treated with bergapten for six days in the presence of LPS, and the area of bone resorption was analyzed with Image Pro-Plus. Next, we examined apoptosis of RAW264.7 cells after bergapten incubation for 48 hours by flow cytometer using annexin V/propidium iodide (PI) double labeling. Finally, osteoclast survival was observed by Hoechst 33342 labeling and Western blotting after bergapten treatment for 24 hours.
Results: Data showed that bergapten (5-40 μmol/L) dose-dependently inhibited LPS-induced osteoclast formation and bone resorption. Treatment with bergapten triggered apoptotic death of osteoclast precursor RAW264.7 cells in a dose-dependent manner. Furthermore, bergapten significantly reduced the survival of mature osteoclast, as demonstrated by emergence of apoptotic nuclei and activation of apoptotic protein caspase 3/9.
Conclusions: These findings suggest that bergapten effectively prevents LPS-induced osteoclastogenesis, bone resorption and survival via apoptotic response of osteoclasts and their precursors. The study identifies bergapten as an inhibitor of osteoclast formation and bone resorption and provides evidence that bergapten might be beneficial as an alternative for prevention and treatment of inflammatory bone loss.
Figures


References
-
- Wada N, Maeda H, Yoshimine Y, et al. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004;35:629–635. doi: 10.1016/j.bone.2004.04.023. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

