Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats
- PMID: 24305818
- PMCID: PMC6618786
- DOI: 10.1523/JNEUROSCI.3041-13.2013
Electrophysiological properties of rostral ventrolateral medulla presympathetic neurons modulated by the respiratory network in rats
Abstract
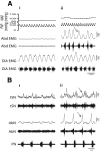
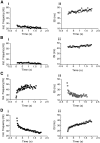
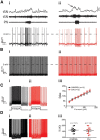
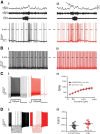
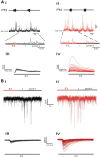
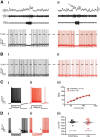
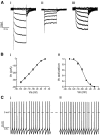
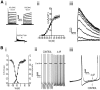
The respiratory pattern generator modulates the sympathetic outflow, the strength of which is enhanced by challenges produced by hypoxia. This coupling is due to the respiratory-modulated presympathetic neurons in the rostral ventrolateral medulla (RVLM), but the underlining electrophysiological mechanisms remain unclear. For a better understanding of the neural substrates responsible for generation of this respiratory-sympathetic coupling, we combined immunofluorescence, single cell qRT-pCR, and electrophysiological recordings of the RVLM presympathetic neurons in in situ preparations from normal rats and rats submitted to a metabolic challenge produced by chronic intermittent hypoxia (CIH). Our results show that the spinally projected cathecholaminergic C1 and non-C1 respiratory-modulated RVLM presympathetic neurons constitute a heterogeneous neuronal population regarding the intrinsic electrophysiological properties, respiratory synaptic inputs, and expression of ionic currents, albeit all neurons presented persistent sodium current-dependent intrinsic pacemaker properties after synaptic blockade. A specific subpopulation of non-C1 respiratory-modulated RVLM presympathetic neurons presented enhanced excitatory synaptic inputs from the respiratory network after CIH. This phenomenon may contribute to the increased sympathetic activity observed in CIH rats. We conclude that the different respiratory-modulated RVLM presympathetic neurons contribute to the central generation of respiratory-sympathetic coupling as part of a complex neuronal network, which in response to the challenges produced by CIH contribute to respiratory-related increase in the sympathetic activity.
Keywords: bulbospinal RVLM presympathetic neurons; chronic intermittent hypoxia; intrinsic electrophysiological properties; respiratory-sympathetic coupling.
Figures





References
-
- Allen AM, Guyenet PG. Alpha 2-adrenoceptor-mediated inhibition of bulbospinal barosensitive cells of rat rostral medulla. Am J Physiol. 1993;265:R1065–R1075. - PubMed
-
- Badra LJ, Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Respiratory modulation of human autonomic rhythms. Am J Physiol Heart Circ Physiol. 2001;280:H2674–2688. - PubMed
-
- Barman SM, Gebber GL. Sympathetic nerve rhythm of brain stem origin. Am J Physiol. 1980;239:R42–R47. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
