Expression of chimeric receptor CD4ζ by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo
- PMID: 24307574
- PMCID: PMC3960346
- DOI: 10.1002/stem.1611
Expression of chimeric receptor CD4ζ by natural killer cells derived from human pluripotent stem cells improves in vitro activity but does not enhance suppression of HIV infection in vivo
Abstract
Cell-based immunotherapy has been gaining interest as an improved means to treat human immunodeficiency virus (HIV)/AIDS. Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) could become a potential resource. Our previous studies have shown hESC and iPSC-derived natural killer (NK) cells can inhibit HIV-infected targets in vitro. Here, we advance those studies by expressing a HIV chimeric receptor combining the extracellular portion of CD4 to the CD3ζ intracellular signaling chain. We hypothesized that expression of this CD4ζ receptor would more efficiently direct hESC- and iPSC-derived NK cells to target HIV-infected cells. In vitro studies showed the CD4ζ expressing hESC- and iPSC-NK cells inhibited HIV replication in CD4+ T-cells more efficiently than their unmodified counterparts. We then evaluated CD4ζ expressing hESC (CD4ζ-hESC)- and iPSC-NK cells in vivo anti-HIV activity using a humanized mouse model. We demonstrated significant suppression of HIV replication in mice treated with both CD4ζ-modified and -unmodified hESC-/iPSC-NK cells compared with control mice. However, we did not observe significantly increased efficacy of CD4ζ expression in suppression of HIV infection. These studies indicate that hESC/iPSC-based immunotherapy can be used as a unique resource to target HIV/AIDS.
Keywords: HIV-1 infection inhibition; Human embryonic stem cells; In vitro; In vivo; Induced pluripotent stem cells; Natural killer cells.
© 2013 AlphaMed Press.
Figures
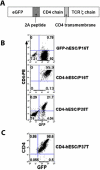

 ) or without (
) or without ( ) anti-CD4 and goat anti-mouse IgG F(ab’)2 to initiate receptor cross-linking. Cells were then intracellular stained by tyrosine phosphorylation Ab 4G10 followed by PE- anti-mouse IgG. Cross-linked cells were stained with mouse IgG and PE- anti-mouse IgG were used as isotype controls (
) anti-CD4 and goat anti-mouse IgG F(ab’)2 to initiate receptor cross-linking. Cells were then intracellular stained by tyrosine phosphorylation Ab 4G10 followed by PE- anti-mouse IgG. Cross-linked cells were stained with mouse IgG and PE- anti-mouse IgG were used as isotype controls ( ). Flow cytometry plots represented 1 of at least 3 independent experiments. (D) Trysine phosphorylation measured by flow cytometry for the mean fluorescent intensity (MFI). The solid lines represent the mean +/− the SD.
). Flow cytometry plots represented 1 of at least 3 independent experiments. (D) Trysine phosphorylation measured by flow cytometry for the mean fluorescent intensity (MFI). The solid lines represent the mean +/− the SD.
 ) and 5:1 (
) and 5:1 ( ). Cells were CEM gated. The error bars represent the mean +/− the standard deviation (SD). Statistical comparison of % GFP+ between CD4ζ-hESC-/iPSC-NK vs. hESC-/iPSC-NK cells was performed using the Student's t test. (C-F) NK cells function against HIV-1-infected human CD4+ primary T cells. (C and D) NK cells were co-cultured with SF2-infected CD4+ T cells at E:T rations of 5:1 for two weeks. HIV infection was evaluated by intracellular staining for gag p24 in all CD4 T cells. The percentage of p24+ CD4+ in the co-cultures of (C) no NKs, PB-, hESC-, and CD4ζ-hESC-NK cells or (D) no NKs PB-, iPSC- and CD4ζ-iPSC-NK cells with HIV-infected CD4 T cells at day 11. Cells were CD56− gated. (C) and (D) demonstrate statistically lower % p24+ in CD4ζ-hESC-/iPSC-NK culture compared to hESC-/iPSC-NK cells respectively. (E) NK cells were evaluated for HIV infection in all CD56+ cells at day 11 of co-culture. Either CD4ζ-hESC-NKs or hESC-NKs were negative for p24 staining. hESC-NKs with no HIV as negative controls. (F and G) Surface expression of CD107a was evaluated to measure NK cell cytolytic activity. Flow cytometric analyses of CD107a expression on (F) hESC- and CD4ζ-hESC-NKs or (G) iPSC- and CD4ζ-iPSC-NKs following stimulation with HIV-1-infected CD4+ T cells for 5 hours. Uninfected CD4+ T cells were used as controls. Cells were all CD56+ gated. Both CD4ζ-hESC- and CD4ζ-iPSC-NK cells populations stimulated by HIV-1-infected CD4+ T cells show significantly increased CD107a expression compared to hESC- and iPSC-NK cells (P<0.05). The data represent one of at least 3 independent experiments.
). Cells were CEM gated. The error bars represent the mean +/− the standard deviation (SD). Statistical comparison of % GFP+ between CD4ζ-hESC-/iPSC-NK vs. hESC-/iPSC-NK cells was performed using the Student's t test. (C-F) NK cells function against HIV-1-infected human CD4+ primary T cells. (C and D) NK cells were co-cultured with SF2-infected CD4+ T cells at E:T rations of 5:1 for two weeks. HIV infection was evaluated by intracellular staining for gag p24 in all CD4 T cells. The percentage of p24+ CD4+ in the co-cultures of (C) no NKs, PB-, hESC-, and CD4ζ-hESC-NK cells or (D) no NKs PB-, iPSC- and CD4ζ-iPSC-NK cells with HIV-infected CD4 T cells at day 11. Cells were CD56− gated. (C) and (D) demonstrate statistically lower % p24+ in CD4ζ-hESC-/iPSC-NK culture compared to hESC-/iPSC-NK cells respectively. (E) NK cells were evaluated for HIV infection in all CD56+ cells at day 11 of co-culture. Either CD4ζ-hESC-NKs or hESC-NKs were negative for p24 staining. hESC-NKs with no HIV as negative controls. (F and G) Surface expression of CD107a was evaluated to measure NK cell cytolytic activity. Flow cytometric analyses of CD107a expression on (F) hESC- and CD4ζ-hESC-NKs or (G) iPSC- and CD4ζ-iPSC-NKs following stimulation with HIV-1-infected CD4+ T cells for 5 hours. Uninfected CD4+ T cells were used as controls. Cells were all CD56+ gated. Both CD4ζ-hESC- and CD4ζ-iPSC-NK cells populations stimulated by HIV-1-infected CD4+ T cells show significantly increased CD107a expression compared to hESC- and iPSC-NK cells (P<0.05). The data represent one of at least 3 independent experiments.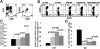


Similar articles
-
Human pluripotent stem cells produce natural killer cells that mediate anti-HIV-1 activity by utilizing diverse cellular mechanisms.J Virol. 2011 Jan;85(1):43-50. doi: 10.1128/JVI.01774-10. Epub 2010 Oct 20. J Virol. 2011. PMID: 20962093 Free PMC article.
-
An Improved Method to Produce Clinical-Scale Natural Killer Cells from Human Pluripotent Stem Cells.Methods Mol Biol. 2019;2048:107-119. doi: 10.1007/978-1-4939-9728-2_12. Methods Mol Biol. 2019. PMID: 31396935
-
Developmental dissociation of T cells from B, NK, and myeloid cells revealed by MHC class II-specific chimeric immune receptors bearing TCR-zeta or FcR-gamma chain signaling domains.Blood. 2002 Oct 15;100(8):3045-8. doi: 10.1182/blood-2002-02-0428. Blood. 2002. PMID: 12351421
-
Development of innate immune cells from human pluripotent stem cells.Exp Hematol. 2019 Mar;71:13-23. doi: 10.1016/j.exphem.2018.12.005. Epub 2019 Jan 4. Exp Hematol. 2019. PMID: 30611869 Free PMC article. Review.
-
Engineered human pluripotent stem cell-derived natural killer cells: the next frontier for cancer immunotherapy.Blood Sci. 2019 Sep 17;1(1):4-11. doi: 10.1097/BS9.0000000000000023. eCollection 2019 Aug. Blood Sci. 2019. PMID: 35402797 Free PMC article. Review.
Cited by
-
Modeling Viral Infectious Diseases and Development of Antiviral Therapies Using Human Induced Pluripotent Stem Cell-Derived Systems.Viruses. 2015 Jul 13;7(7):3835-56. doi: 10.3390/v7072800. Viruses. 2015. PMID: 26184286 Free PMC article. Review.
-
Chimeric antigen receptor T-cell approaches to HIV cure.Curr Opin HIV AIDS. 2018 Sep;13(5):446-453. doi: 10.1097/COH.0000000000000485. Curr Opin HIV AIDS. 2018. PMID: 29878913 Free PMC article. Review.
-
Advances in Induced Pluripotent Stem Cell-Derived Natural Killer Cell Therapy.Cells. 2024 Nov 29;13(23):1976. doi: 10.3390/cells13231976. Cells. 2024. PMID: 39682724 Free PMC article. Review.
-
Prospects for Development of Induced Pluripotent Stem Cell-Derived CAR-Targeted Immunotherapies.Arch Immunol Ther Exp (Warsz). 2021 Dec 12;70(1):2. doi: 10.1007/s00005-021-00640-7. Arch Immunol Ther Exp (Warsz). 2021. PMID: 34897554 Free PMC article. Review.
-
Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells.PLoS One. 2015 Mar 27;10(3):e0121788. doi: 10.1371/journal.pone.0121788. eCollection 2015. PLoS One. 2015. PMID: 25816339 Free PMC article.
References
-
- Fauci AS, Mavilio D, Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. NATURE REVIEWS. 2005;5:835–843. - PubMed
-
- Iannello A, Boulassel MR, Samarani S, et al. Dynamics and consequences of IL-21 production in HIV-infected individuals: a longitudinal and cross-sectional study. JOURNAL OF IMMUNOLOGY. 2010;184:114–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

