A link between gut community metabolism and pathogenesis: molecular hydrogen-stimulated glucarate catabolism aids Salmonella virulence
- PMID: 24307595
- PMCID: PMC3877842
- DOI: 10.1098/rsob.130146
A link between gut community metabolism and pathogenesis: molecular hydrogen-stimulated glucarate catabolism aids Salmonella virulence
Abstract
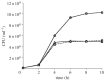
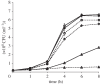
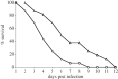
Glucarate, an oxidized product of glucose, is a major serum organic acid in humans. Still, its role as a carbon source for a pathogen colonizing hosts has not been studied. We detected high-level expression of a potential glucarate permease encoding gene gudT when Salmonella enterica serovar Typhimurium are exposed to hydrogen gas (H(2)), a gaseous by-product of gut commensal metabolism. A gudT strain of Salmonella is deficient in glucarate-dependent growth, however, it can still use other monosaccharides, such as glucose or galactose. Complementation of the gudT mutant with a plasmid harbouring gudT restored glucarate-dependent growth to wild-type (WT) levels. The gudT mutant exhibits attenuated virulence: the mean time of death for mice inoculated with WT strain was 2 days earlier than for mice inoculated with the gudT strain. At 4 days postinoculation, liver and spleen homogenates from mice inoculated with a gudT strain contained significantly fewer viable Salmonella than homogenates from animals inoculated with the parent. The parent strain grew well H(2)-dependently in a minimal medium with amino acids and glucarate provided as the sole carbon sources, whereas the gudT strain achieved approximately 30% of the parent strain's yield. Glucarate-mediated growth of a mutant strain unable to produce H(2) was stimulated by H(2) addition, presumably owing to the positive transcriptional response to H(2). Gut microbiota-produced molecular hydrogen apparently signals Salmonella to catabolize an alternative carbon source available in the host. Our results link a gut microbiome-produced diffusible metabolite to augmenting bacterial pathogenesis.
Keywords: carbon transport; gut microbiome; in vivo pathogen growth; metabolism and virulence; microbial carbon utilization.
Figures
Similar articles
-
Host hydrogen rather than that produced by the pathogen is important for Salmonella enterica serovar Typhimurium virulence.Infect Immun. 2015 Jan;83(1):311-6. doi: 10.1128/IAI.02611-14. Epub 2014 Nov 3. Infect Immun. 2015. PMID: 25368112 Free PMC article.
-
Respiratory hydrogen use by Salmonella enterica serovar Typhimurium is essential for virulence.Infect Immun. 2004 Nov;72(11):6294-9. doi: 10.1128/IAI.72.11.6294-6299.2004. Infect Immun. 2004. PMID: 15501756 Free PMC article.
-
Enterobacterial common antigen mutants of Salmonella enterica serovar Typhimurium establish a persistent infection and provide protection against subsequent lethal challenge.Infect Immun. 2012 Jan;80(1):441-50. doi: 10.1128/IAI.05559-11. Epub 2011 Oct 24. Infect Immun. 2012. PMID: 22025511 Free PMC article.
-
Molecular pathogenesis of Salmonella enterica serotype typhimurium-induced diarrhea.Infect Immun. 2003 Jan;71(1):1-12. doi: 10.1128/IAI.71.1.1-12.2003. Infect Immun. 2003. PMID: 12496143 Free PMC article. Review. No abstract available.
-
Virulence Factors in Salmonella Typhimurium: The Sagacity of a Bacterium.Curr Microbiol. 2019 Jun;76(6):762-773. doi: 10.1007/s00284-018-1510-4. Epub 2018 May 21. Curr Microbiol. 2019. PMID: 29785632 Review.
Cited by
-
Microbiology: The dark side of antibiotics.Nature. 2016 Jun 30;534(7609):624-5. doi: 10.1038/nature18449. Epub 2016 Jun 15. Nature. 2016. PMID: 27309812 No abstract available.
-
How Bacterial Pathogens Coordinate Appetite with Virulence.Microbiol Mol Biol Rev. 2023 Sep 26;87(3):e0019822. doi: 10.1128/mmbr.00198-22. Epub 2023 Jun 26. Microbiol Mol Biol Rev. 2023. PMID: 37358444 Free PMC article. Review.
-
Effect of diet on pathogen performance in the microbiome.Microbiome Res Rep. 2022 Mar 26;1(2):13. doi: 10.20517/mrr.2021.10. eCollection 2022. Microbiome Res Rep. 2022. PMID: 38045644 Free PMC article. Review.
-
Integrated OMICs approach reveals energy metabolism pathway is vital for Salmonella Pullorum survival within the egg white.mSphere. 2024 Jul 30;9(7):e0036224. doi: 10.1128/msphere.00362-24. Epub 2024 Jun 11. mSphere. 2024. PMID: 38860771 Free PMC article.
-
Molecular Hydrogen Metabolism: a Widespread Trait of Pathogenic Bacteria and Protists.Microbiol Mol Biol Rev. 2020 Jan 29;84(1):e00092-19. doi: 10.1128/MMBR.00092-19. Print 2020 Feb 19. Microbiol Mol Biol Rev. 2020. PMID: 31996394 Free PMC article. Review.
References
-
- Anet EFJ, Reynolds TM. 1954. Isolation of mucic acid from fruits. Nature 174, 930–932 (doi:10.1038/174930a0) - DOI - PubMed
-
- Whiting GC, Coggins RA. 1960. Organic acid metabolism in cider and perry fermentations. III. Keto-acids in cider-apple juices and ciders. J. Sci. Food. Agric. 11, 337–341 (doi:10.1002/jsfa.2740110608) - DOI
-
- Marsh CA. 1985. An enzymatic determination of d-glucaric acid by conversion to pyruvate. Anal. Biochem. 145, 266–272 (doi:10.1016/0003-2697(85)90360-4) - DOI - PubMed
-
- Matsui M, Fukuo A, Watanabe Y, Wanibe T, Okada M. 1977. Studies on the glucaric acid pathway in the metabolism of d-glucuronic acid in mammals. IV. Fluorometric method for the determination of glucaric acid in serum. Chem. Pharm. Bull. 20, 845–848 (doi:10.1248/cpb.20.845) - DOI - PubMed
-
- Dutton GJ. 1980. Glucuronidation of drugs and other compounds, pp. 83–89 Boca Raton, FL: CRC Press
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

