Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells
- PMID: 24307611
- PMCID: PMC3889522
- DOI: 10.1208/s12248-013-9550-y
Squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells
Erratum in
-
Erratum to: squarticles as a lipid nanocarrier for delivering diphencyprone and minoxidil to hair follicles and human dermal papilla cells.AAPS J. 2015 Mar;17(2):480. doi: 10.1208/s12248-014-9692-6. AAPS J. 2015. PMID: 25351605 Free PMC article. No abstract available.
Abstract
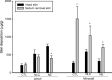
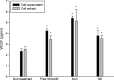
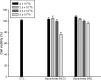
Delivery of diphencyprone (DPCP) and minoxidil to hair follicles and related cells is important in the treatment of alopecia. Here we report the development of "squarticles," nanoparticles formed from sebum-derived lipids such as squalene and fatty esters, for use in achieving targeted drug delivery to the follicles. Two different nanosystems, nanostructured lipid carriers (NLC) and nanoemulsions (NE), were prepared. The physicochemical properties of squarticles, including size, zeta potential, drug encapsulation efficiency, and drug release, were examined. Squarticles were compared to a free control solution with respect to skin absorption, follicular accumulation, and dermal papilla cell targeting. The particle size of the NLC type was 177 nm; that of the NE type was 194 nm. Approximately 80% of DPCP and 60% of minoxidil were entrapped into squarticles. An improved drug deposition in the skin was observed in the in vitro absorption test. Compared to the free control, the squarticles reduced minoxidil penetration through the skin. This may indicate a minimized absorption into systemic circulation. Follicular uptake by squarticles was 2- and 7-fold higher for DPCP and minoxidil respectively compared to the free control. Fluorescence and confocal images of the skin confirmed a great accumulation of squarticles in the follicles and the deeper skin strata. Vascular endothelial growth factor expression in dermal papilla cells was significantly upregulated after the loading of minoxidil into the squarticles. In vitro papilla cell viability and in vivo skin irritancy tests in nude mice suggested a good tolerability of squarticles to skin. Squarticles provide a promising nanocarrier for topical delivery of DPCP and minoxidil.
Figures




Similar articles
-
Anti-PDGF receptor β antibody-conjugated squarticles loaded with minoxidil for alopecia treatment by targeting hair follicles and dermal papilla cells.Nanomedicine. 2015 Aug;11(6):1321-30. doi: 10.1016/j.nano.2015.04.009. Epub 2015 Apr 29. Nanomedicine. 2015. PMID: 25933696
-
Squalene-containing nanostructured lipid carriers promote percutaneous absorption and hair follicle targeting of diphencyprone for treating alopecia areata.Pharm Res. 2013 Feb;30(2):435-46. doi: 10.1007/s11095-012-0888-0. Epub 2012 Oct 16. Pharm Res. 2013. PMID: 23070602
-
Minoxidil-encapsulated poly(L-lactide-co-glycolide) nanoparticles with hair follicle delivery properties prepared using W/O/W solvent evaporation and sonication.Biomed Mater Eng. 2018;29(2):217-228. doi: 10.3233/BME-171724. Biomed Mater Eng. 2018. PMID: 29457595
-
An update on nanocarriers for follicular-targeted drug delivery for androgenetic alopecia topical treatment.Expert Opin Drug Deliv. 2025 Mar;22(3):367-381. doi: 10.1080/17425247.2025.2457950. Epub 2025 Jan 25. Expert Opin Drug Deliv. 2025. PMID: 39841606 Review.
-
Applications and limitations of lipid nanoparticles in dermal and transdermal drug delivery via the follicular route.Eur J Pharm Biopharm. 2015 Nov;97(Pt A):152-63. doi: 10.1016/j.ejpb.2015.06.020. Epub 2015 Jul 2. Eur J Pharm Biopharm. 2015. PMID: 26144664 Review.
Cited by
-
Oleic Acid Nanovesicles of Minoxidil for Enhanced Follicular Delivery.Medicines (Basel). 2018 Sep 14;5(3):103. doi: 10.3390/medicines5030103. Medicines (Basel). 2018. PMID: 30223446 Free PMC article.
-
Selective Delivery of Tofacitinib Citrate to Hair Follicles Using Lipid-Coated Calcium Carbonate Nanocarrier Controls Chemotherapy-Induced Alopecia Areata.Int J Mol Sci. 2023 May 8;24(9):8427. doi: 10.3390/ijms24098427. Int J Mol Sci. 2023. PMID: 37176141 Free PMC article.
-
Betulin-NLC-hydrogel for the Treatment of Psoriasis-like Skin Inflammation: Optimization, Characterisation, and In vitro and In vivo Evaluation.Curr Drug Deliv. 2025;22(5):627-647. doi: 10.2174/0115672018329544240922151617. Curr Drug Deliv. 2025. PMID: 39360545
-
Platycarya strobilacea S. et Z. Extract Has a High Antioxidant Capacity and Exhibits Hair Growth-promoting Effects in Male C57BL/6 Mice.Prev Nutr Food Sci. 2014 Sep;19(3):136-44. doi: 10.3746/pnf.2014.19.3.136. Prev Nutr Food Sci. 2014. PMID: 25320710 Free PMC article.
-
Effects of Natural Polyphenols on Skin and Hair Health: A Review.Molecules. 2022 Nov 14;27(22):7832. doi: 10.3390/molecules27227832. Molecules. 2022. PMID: 36431932 Free PMC article. Review.
References
-
- Tsuboi R, Itami S, Inui S, Ueki R, Katsuoka K, Kurata S, et al. Guidelines for the management of androgenetic alopecia (2010) J Dermatol. 2011;38:1–8. doi: 10.1111/j.1346-8138.2010.01198.x. - DOI
-
- Pray WS. Prevention and treatment of hair loss in men. US Pharmasist. 2008;33:16–20.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

