Recurrent gene loss correlates with the evolution of stomach phenotypes in gnathostome history
- PMID: 24307675
- PMCID: PMC3866411
- DOI: 10.1098/rspb.2013.2669
Recurrent gene loss correlates with the evolution of stomach phenotypes in gnathostome history
Abstract
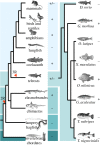
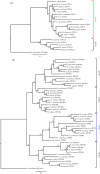
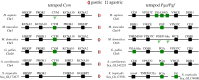
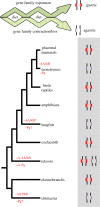
The stomach, a hallmark of gnathostome evolution, represents a unique anatomical innovation characterized by the presence of acid- and pepsin-secreting glands. However, the occurrence of these glands in gnathostome species is not universal; in the nineteenth century the French zoologist Cuvier first noted that some teleosts lacked a stomach. Strikingly, Holocephali (chimaeras), dipnoids (lungfish) and monotremes (egg-laying mammals) also lack acid secretion and a gastric cellular phenotype. Here, we test the hypothesis that loss of the gastric phenotype is correlated with the loss of key gastric genes. We investigated species from all the main gnathostome lineages and show the specific contribution of gene loss to the widespread distribution of the agastric condition. We establish that the stomach loss correlates with the persistent and complete absence of the gastric function gene kit--H(+)/K(+)-ATPase (Atp4A and Atp4B) and pepsinogens (Pga, Pgc, Cym)--in the analysed species. We also find that in gastric species the pepsinogen gene complement varies significantly (e.g. two to four in teleosts and tens in some mammals) with multiple events of pseudogenization identified in various lineages. We propose that relaxation of purifying selection in pepsinogen genes and possibly proton pump genes in response to dietary changes led to the numerous independent events of stomach loss in gnathostome history. Significantly, the absence of the gastric genes predicts that reinvention of the stomach in agastric lineages would be highly improbable, in line with Dollo's principle.
Keywords: gene duplication; gene loss; gnathostomes; pepsinogen; proton pump; stomach.
Figures


Similar articles
-
Generation of gastric proton pump atp4a knockouts in Astyanax mexicanus: a fish model for insights into the mechanisms of acidification by oxynticopeptic cells.Am J Physiol Gastrointest Liver Physiol. 2025 Jul 1;329(1):G88-G101. doi: 10.1152/ajpgi.00382.2024. Epub 2025 May 15. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 40372704
-
The Gastric Phenotype in the Cypriniform Loaches: A Case of Reinvention?PLoS One. 2016 Oct 26;11(10):e0163696. doi: 10.1371/journal.pone.0163696. eCollection 2016. PLoS One. 2016. PMID: 27783698 Free PMC article.
-
The gastric proton pump in gobiid and mudskipper fishes. Evidence of stomach loss?Comp Biochem Physiol A Mol Integr Physiol. 2022 Dec;274:111300. doi: 10.1016/j.cbpa.2022.111300. Epub 2022 Aug 27. Comp Biochem Physiol A Mol Integr Physiol. 2022. PMID: 36031062
-
[Gastric proton pump: genes and their expression].Nihon Rinsho. 1996 Apr;54(4):1138-43. Nihon Rinsho. 1996. PMID: 8920687 Review. Japanese.
-
Pepsinogens: physiology, pharmacology pathophysiology and exercise.Pharmacol Res. 2000 Mar;41(3):265-81. doi: 10.1006/phrs.1999.0586. Pharmacol Res. 2000. PMID: 10675278 Review.
Cited by
-
Evolution by gene loss.Nat Rev Genet. 2016 Jul;17(7):379-91. doi: 10.1038/nrg.2016.39. Epub 2016 Apr 18. Nat Rev Genet. 2016. PMID: 27087500 Review.
-
Co-option of immune and digestive cellular machinery to support photosymbiosis in amoebocytes of the upside-down jellyfish Cassiopea xamachana.J Exp Biol. 2025 Jul 15;228(14):jeb249849. doi: 10.1242/jeb.249849. Epub 2025 May 12. J Exp Biol. 2025. PMID: 40110628 Free PMC article.
-
A time-resolved multi-omic atlas of the developing mouse stomach.Nat Commun. 2018 Nov 21;9(1):4910. doi: 10.1038/s41467-018-07463-9. Nat Commun. 2018. PMID: 30464175 Free PMC article.
-
Cross-species single-cell transcriptomic analysis of animal gastric antrum reveals intense porcine mucosal immunity.Cell Regen. 2023 Aug 1;12(1):27. doi: 10.1186/s13619-023-00171-w. Cell Regen. 2023. PMID: 37525021 Free PMC article.
-
Loss of stomach, loss of appetite? Sequencing of the ballan wrasse (Labrus bergylta) genome and intestinal transcriptomic profiling illuminate the evolution of loss of stomach function in fish.BMC Genomics. 2018 Mar 6;19(1):186. doi: 10.1186/s12864-018-4570-8. BMC Genomics. 2018. PMID: 29510660 Free PMC article.
References
-
- Ohno S. 1970. Evolution by gene duplication. Berlin, Germany: Springer
-
- Shimeld SM, Holland PW. 2000. Vertebrate innovations. Proc. Natl Acad. Sci. USA 97, 4449–4452 (doi:10.1073/pnas.97.9.4449) - DOI - PMC - PubMed
-
- Braasch I, Brunet F, Volff JN, Schartl M. 2009. Pigmentation pathway evolution after whole-genome duplication in fish. Genome Biol. Evol. 1, 479–493 (doi:10.1093/gbe/evp050) - DOI - PMC - PubMed
-
- Widmark J, Sundström G, Ocampo Daza D, Larhammar D. 2011. Differential evolution of voltage-gated sodium channels in tetrapods and teleost fishes. Mol. Biol. Evol. 28, 859–871 (doi:10.1093/molbev/msq257) - DOI - PubMed
-
- Hoffmann FG, Opazo JC, Storz JF. 2012. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol. Biol. Evol. 29, 303–312 (doi:10.1093/molbev/msr207) - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

