MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3'-5')-cyclic-di-guanosine-monophosphate in vivo
- PMID: 24307739
- PMCID: PMC6195764
- DOI: 10.4049/jimmunol.1301812
MPYS/STING-mediated TNF-α, not type I IFN, is essential for the mucosal adjuvant activity of (3'-5')-cyclic-di-guanosine-monophosphate in vivo
Abstract
The bacterial second messenger (3'-5')-cyclic-di-guanosine-monophosphate (CDG) is a promising mucosal adjuvant candidate that activates balanced Th1/Th2/Th17 responses. We showed previously that CDG activates stimulator of IFN genes (STING)-dependent IFN-I production in vitro. However, it is unknown whether STING or IFN-I is required for the CDG adjuvant activity in vivo. In this study, we show that STING(-/-) mice (Tmem173(<tm1Camb>)) do not produce Ag-specific Abs or Th1/Th2/Th17 cytokines during CDG/Ag immunization. Intranasal administration of CDG did not induce TNF-α, IL-1β, IL-6, IL-12, or MCP-1 production in STING(-/-) mice. Surprisingly, we found that the cytokine and Ab responses were unaltered in CDG/Ag-immunized IFNAR(-/-) mice. Instead, we found that CDG activates STING-dependent, IFN-I-independent TNF-α production in vivo and in vitro. Furthermore, using a TNFR1(-/-) mouse, we demonstrate that TNF-α signaling is critical for CDG-induced Ag-specific Ab and Th1/Th2 cytokine production. This is distinct from STING-mediated DNA adjuvant activity, which requires IFN-I, but not TNF-α, production. Finally, we found that CDG activates STING-dependent, but IRF3 stimulation-independent, NF-κB signaling. Our results established an essential role for STING-mediated TNF-α production in the mucosal adjuvant activity of CDG in vivo and revealed a novel IFN-I stimulation-independent STING-NF-κB-TNF-α pathway.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures

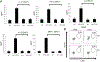


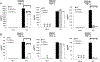

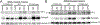


References
-
- Karaolis DK, Means TK, Yang D, Takahashi M, Yoshimura T, Muraille E,Philpott D, Schroeder JT, Hyodo M, Hayakawa Y, et al. 2007. Bacterial c-di-GMP is an immunostimulatory molecule. J. Immunol 178: 2171–2181. - PubMed
-
- Ebensen T, Schulze K, Riese P, Link C, Morr M, and Guzma CA´n. 2007. The bacterial second messenger cyclic diGMP exhibits potent adjuvant properties. Vaccine 25: 1464–1469. - PubMed
-
- Madhun AS, Haaheim LR, Nøstbakken JK, Ebensen T, Chichester J,Yusibov V, Guzman CA, and Cox RJ 2011. Intranasal c-di-GMP-adjuvanted plant-derived H5 influenza vaccine induces multifunctional Th1 CD4+ cells and strong mucosal and systemic antibody responses in mice. Vaccine 29: 4973–4982. - PubMed
-
- Zhao L, KuoLee R, Harris G, Tram K, Yan H, and Chen W 2011. c-di-GMPprotects against intranasal Acinetobacter baumannii infection in mice by chemokine induction and enhanced neutrophil recruitment. Int. Immunopharmacol 11: 1378–1383. - PubMed
-
- Hu DL, Narita K, Hyodo M, Hayakawa Y, Nakane A, and Karaolis DK 2009. c-di-GMP as a vaccine adjuvant enhances protection against systemic methicillin-resistant Staphylococcus aureus (MRSA) infection. Vaccine 27: 4867–4873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

