Drought-induced trans-generational tradeoff between stress tolerance and defence: consequences for range limits?
- PMID: 24307931
- PMCID: PMC3849778
- DOI: 10.1093/aobpla/plt038
Drought-induced trans-generational tradeoff between stress tolerance and defence: consequences for range limits?
Abstract
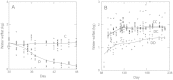
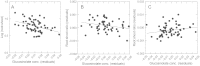
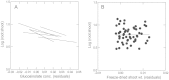
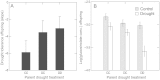
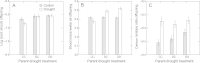
Areas just across species range boundaries are often stressful, but even with ample genetic variation within and among range-margin populations, adaptation towards stress tolerance across range boundaries often does not occur. Adaptive trans-generational plasticity should allow organisms to circumvent these problems for temporary range expansion; however, range boundaries often persist. To investigate this dilemma, we drought stressed a parent generation of Boechera stricta (A.Gray) A. Löve & D. Löve, a perennial wild relative of Arabidopsis, representing genetic variation within and among several low-elevation range margin populations. Boechera stricta is restricted to higher, moister elevations in temperate regions where generalist herbivores are often less common. Previous reports indicate a negative genetic correlation (genetic tradeoff) between chemical defence allocation and abiotic stress tolerance that may prevent the simultaneous evolution of defence and drought tolerance that would be needed for range expansion. In growth chamber experiments, the genetic tradeoff became undetectable among offspring sib-families whose parents had been drought treated, suggesting that the stress-induced trans-generational plasticity may circumvent the genetic tradeoff and thus enable range expansion. However, the trans-generational effects also included a conflict between plastic responses (environmental tradeoff); offspring whose parents were drought treated were more drought tolerant, but had lower levels of glucosinolate toxins that function in defence against generalist herbivores. We suggest that either the genetic or environmental tradeoff between defence allocation and stress tolerance has the potential to contribute to range limit development in upland mustards.
Keywords: Boechera stricta; chemical defence; drought tolerance; epigenetic; glucosinolate; range limit; tradeoff.
Figures
References
-
- Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, Mansurova M, Humphry M, Consonni C, Panstruga R, Sanchez-Vallet A, Molina A, Schulze-Lefert P. A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science. 2009;323:101–106. - PubMed
-
- Brown P, Tokuhisa J, Reichelt M, Gershenzon J. Variation of glucosinolate accumulation among different organs and developmental stages of Arabidopsis thaliana. Phytochemistry. 2003;62:471–481. - PubMed
-
- Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Phytochemistry and Photobiology. 1999;70:1–9.
-
- Conner JK, Hartl DL. A primer of ecological genetics. Sunderland: MA: Sinauer; 2004.
LinkOut - more resources
Full Text Sources
Other Literature Sources

