Effectiveness and safety of intensive triplet chemotherapy plus bevacizumab, FIr-B/FOx, in young-elderly metastatic colorectal cancer patients
- PMID: 24307987
- PMCID: PMC3838846
- DOI: 10.1155/2013/143273
Effectiveness and safety of intensive triplet chemotherapy plus bevacizumab, FIr-B/FOx, in young-elderly metastatic colorectal cancer patients
Abstract
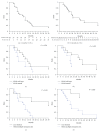
Four-drug regimens, such as FIr-B/FOx schedule, can improve efficacy of first-line treatment of metastatic colorectal cancer (MCRC) patients. The present study specifically evaluates feasibility of FIr-B/FOx first-line intensive regimen in fit young-elderly MCRC patients, representing approximately 40% of overall MCRC patients. Activity, efficacy, and safety were equivalent to overall MCRC patients, not significantly different according to KRAS genotype. Clinical outcome was significantly prolonged in liver-limited compared to other/multiple metastatic disease. Safety evaluation of the individual young-elderly patient showed that limiting toxicity syndromes (LTS) in multiple sites were significantly increased, compared to LTS in single site, with respect to non-elderly patients.
Figures
Similar articles
-
Differential prognosis of metastatic colorectal cancer patients post-progression to first-line triplet chemotherapy plus bevacizumab, FIr-B/FOx, according to second-line treatment and KRAS genotype.Int J Oncol. 2014 Jan;44(1):17-26. doi: 10.3892/ijo.2013.2179. Epub 2013 Nov 15. Int J Oncol. 2014. PMID: 24247407 Free PMC article.
-
Prognostic value of KRAS genotype in metastatic colorectal cancer (MCRC) patients treated with intensive triplet chemotherapy plus bevacizumab (FIr-B/FOx) according to extension of metastatic disease.BMC Med. 2012 Nov 8;10:135. doi: 10.1186/1741-7015-10-135. BMC Med. 2012. PMID: 23136868 Free PMC article.
-
Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial.World J Gastroenterol. 2010 Jul 7;16(25):3133-43. doi: 10.3748/wjg.v16.i25.3133. World J Gastroenterol. 2010. PMID: 20593498 Free PMC article. Clinical Trial.
-
Sequencing of antiangiogenic agents in the treatment of metastatic colorectal cancer.Clin Colorectal Cancer. 2014 Sep;13(3):135-44. doi: 10.1016/j.clcc.2014.02.001. Epub 2014 Feb 27. Clin Colorectal Cancer. 2014. PMID: 24768040 Review.
-
The prevalent KRAS exon 2 c.35 G>A mutation in metastatic colorectal cancer patients: A biomarker of worse prognosis and potential benefit of bevacizumab-containing intensive regimens?Crit Rev Oncol Hematol. 2015 Mar;93(3):190-202. doi: 10.1016/j.critrevonc.2014.10.004. Epub 2014 Oct 16. Crit Rev Oncol Hematol. 2015. PMID: 25459669 Review.
Cited by
-
Dose-finding study of oxaliplatin associated to capecitabine-based preoperative chemoradiotherapy in locally advanced rectal cancer.Oncotarget. 2018 Apr 3;9(25):17906-17914. doi: 10.18632/oncotarget.24665. eCollection 2018 Apr 3. Oncotarget. 2018. PMID: 29707156 Free PMC article.
-
Toxicity Syndromes, Patient-Related Clinical Indicator of Toxicity Burden Induced by Intensive Triplet Chemotherapy-Based Regimens in Gastrointestinal Cancers With Metastatic Disease.Front Oncol. 2020 Feb 20;10:172. doi: 10.3389/fonc.2020.00172. eCollection 2020. Front Oncol. 2020. PMID: 32154172 Free PMC article.
-
PSA as the driving biomarker to manage low- and intermediate-risk prostate cancer patients in clinical practice.Asian J Androl. 2024 Nov 1;26(6):567-568. doi: 10.4103/aja202468. Epub 2024 Sep 27. Asian J Androl. 2024. PMID: 39327840 Free PMC article. No abstract available.
-
Prognostic relevance of KRAS genotype in metastatic colorectal cancer patients unfit for FIr-B/FOx intensive regimen.Int J Oncol. 2014 Jun;44(6):1820-30. doi: 10.3892/ijo.2014.2369. Epub 2014 Apr 4. Int J Oncol. 2014. PMID: 24715238 Free PMC article.
-
Multidisciplinary palliation for unresectable recurrent rectal cancer: hypoxic pelvic perfusion with mitomycin C and oxaliplatin in patients progressing after systemic chemotherapy and radiotherapy, a retrospective cohort study.Oncotarget. 2019 Jun 11;10(39):1-13. doi: 10.18632/oncotarget.26972. eCollection 2019 Jun 11. Oncotarget. 2019. PMID: 31231460 Free PMC article.
References
-
- Bruera G, Ricevuto E. Intensive chemotherapy of metastatic colorectal cancer: weighing between safety and clinical efficacy. Evaluation of Masi G, Loupakis F, Salvatore L, et al. Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol 2010;11:845–52. Expert Opinion on Bioogical Theraphy. 2011;11(6):821–824. - PubMed
-
- Bruera G, Cannita K, Giuliante F, et al. Effectiveness of liver metastasectomies in patients with metastatic colorectal cancer treated with FIr-B/FOx triplet chemotherapy plus bevacizumab. Clinical Colorectal Cancer. 2012;11(2):119–126. - PubMed
-
- Ficorella C, Bruera G, Cannita K, et al. Triplet chemotherapy in patients with metastatic colorectal cancer: toward the best way to safely administer a highly active regimen in clinical practice. Clinical Colorectal Cancer. 2012;11(4):229–237. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

