Safety of Porcine Reproductive and Respiratory Syndrome Modified Live Virus (MLV) vaccine strains in a young pig infection model
- PMID: 24308693
- PMCID: PMC4028782
- DOI: 10.1186/1297-9716-44-115
Safety of Porcine Reproductive and Respiratory Syndrome Modified Live Virus (MLV) vaccine strains in a young pig infection model
Abstract
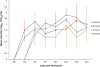
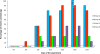
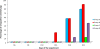
The objective of this study was to compare the safety of all modified live virus vaccines commercially available in Europe against Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) under the same experimental conditions. For this purpose, one hundred and twenty three-week-old piglets, divided into five groups, were used. On day 0 of the experiment, nine pigs per group were removed and the remaining fifteen were vaccinated with the commercial vaccines Ingelvac PRRS MLV, Amervac PRRS, Pyrsvac-183 and Porcilis PRRS by the IM route or were mock vaccinated and used as controls. On day 3, the nine unvaccinated pigs were re-introduced into their respective groups and served as sentinel pigs. Clinical signs were recorded daily and lung lesions were determined on days 7, 14 and 21, when 5 vaccinated pigs per group were euthanized. Blood samples and swabs were taken every three days and different organs were collected at necropsy to determine the presence of PRRSV. None of the vaccines studied caused detectable clinical signs in vaccinated pigs although lung lesions were found. Altogether, these results indicate that all vaccines can be considered clinically safe. However, some differences were found in virological parameters. Thus, neither Pyrsvac-183 nor Porcilis PRRS could be detected in porcine alveolar macrophage (PAM) cultures or in lung sections used to determine PRRSV by immunohistochemistry, indicating that these viruses might have lost their ability to replicate in PAM. This inability to replicate in PAM might be related to the lower transmission rate and the delay in the onset of viremia observed in these groups.
Figures

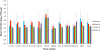

References
-
- Mengeling WL, Lager KM, Vorwald AC. Temporal characterization of transplacental infection of porcine fetuses with porcine reproductive and respiratory syndrome virus. Am J Vet Res. 1994;44:1391–1398. - PubMed
-
- Cavanagh D. Nidovirales: a new order comprising Coronaviridae and Arteriviridae. Arch Virol. 1997;44:629–633. - PubMed
-
- Halbur PG, Paul PS, Frey ML, Landgraf J, Eernisse K, Meng XJ, Lum MA, Andrews JJ, Rathje JA. Comparison of the pathogenicity of two US porcine reproductive and respiratory syndrome virus isolates with that of the Lelystad virus. Vet Pathol. 1995;44:648–660. doi: 10.1177/030098589503200606. - DOI - PubMed
-
- Martínez-Lobo FJ, Díez-Fuertes F, Segalés J, García-Artiga C, Simarro I, Castro JM, Prieto C. Comparative pathogenicity of type 1 and type 2 isolates of porcine reproductive and respiratory syndrome virus (PRRSV) in a young pig infection model. Vet Microbiol. 2011;44:58–68. doi: 10.1016/j.vetmic.2011.06.025. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

