LE135, a retinoid acid receptor antagonist, produces pain through direct activation of TRP channels
- PMID: 24308840
- PMCID: PMC3954489
- DOI: 10.1111/bph.12543
LE135, a retinoid acid receptor antagonist, produces pain through direct activation of TRP channels
Abstract
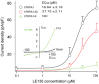
Background and purpose: Retinoids, through their activation of retinoic acid receptors (RARs) and retinoid X receptors, regulate diverse cellular processes, and pharmacological intervention in their actions has been successful in the treatment of skin disorders and cancers. Despite the many beneficial effects, administration of retinoids causes irritating side effects with unknown mechanisms. Here, we demonstrate that LE135 [4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)benzoic acid], a selective antagonist of RARβ , is a potent activator of the capsaicin (TRPV1) and wasabi (TRPA1) receptors, two critical pain-initiating cation channels.
Experimental approach: We performed to investigate the excitatory effects of LE135 on TRPV1 and TRPA1 channels expressed in HEK293T cells and in dorsal root ganglia neurons with calcium imaging and patch-clamp recordings. We also used site-directed mutagenesis of the channels to determine the structural basis of LE135-induced activation of TRPV1 and TRPA1 channels and behavioural testing to examine if pharmacological inhibition and genetic deletion of the channels affected LE135-evoked pain-related behaviours.
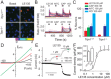
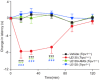
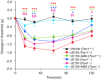
Key results: LE135 activated both the capsaicin receptor (TRPV1) and the allyl isothiocyanate receptor (TRPA1) heterologously expressed in HEK293T cells and endogenously expressed by sensory nociceptors. Mutations disrupting the capsaicin-binding site attenuated LE135 activation of TRPV1 channels and a single mutation (K170R) eliminated TRPA1 activity evoked by LE135. Intraplantar injection of LE135 evoked pain-related behaviours. Both TRPV1 and TRPA1 channels were involved in LE135-elicited pain-related responses, as shown by pharmacological and genetic ablation studies.
Conclusions and implications: This blocker of retinoid acid signalling also exerted non-genomic effects through activating the pain-initiating TRPV1 and TRPA1 channels.
Keywords: LE135; TRPA1; TRPV1; mechanical allodynia; nociception; thermal hyperalgesia.
© 2013 The British Pharmacological Society.
Figures



Similar articles
-
Pronociceptive response elicited by TRPA1 receptor activation in mice.Neuroscience. 2008 Mar 18;152(2):511-20. doi: 10.1016/j.neuroscience.2007.12.039. Epub 2008 Jan 9. Neuroscience. 2008. PMID: 18272293
-
The peptide Phα1β, from spider venom, acts as a TRPA1 channel antagonist with antinociceptive effects in mice.Br J Pharmacol. 2017 Jan;174(1):57-69. doi: 10.1111/bph.13652. Epub 2016 Nov 28. Br J Pharmacol. 2017. PMID: 27759880 Free PMC article.
-
Activation characteristics of transient receptor potential ankyrin 1 and its role in nociception.Am J Physiol Cell Physiol. 2011 Sep;301(3):C587-600. doi: 10.1152/ajpcell.00465.2010. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653898 Free PMC article.
-
[Retinoid antagonists].Yakugaku Zasshi. 1996 Dec;116(12):928-41. doi: 10.1248/yakushi1947.116.12_928. Yakugaku Zasshi. 1996. PMID: 8993231 Review. Japanese.
-
[Activation and regulation of nociceptive transient receptor potential (TRP) channels, TRPV1 and TRPA1].Yakugaku Zasshi. 2010 Mar;130(3):289-94. doi: 10.1248/yakushi.130.289. Yakugaku Zasshi. 2010. PMID: 20190512 Review. Japanese.
Cited by
-
Comprehensive pan‑cancer analysis of potassium voltage-gated channel Q4 (KCNQ4) gene across multiple human malignant tumors.Sci Rep. 2023 Oct 30;13(1):18608. doi: 10.1038/s41598-023-45074-7. Sci Rep. 2023. PMID: 37903775 Free PMC article.
-
Eact, a small molecule activator of TMEM16A, activates TRPV1 and elicits pain- and itch-related behaviours.Br J Pharmacol. 2016 Apr;173(7):1208-18. doi: 10.1111/bph.13420. Epub 2016 Mar 1. Br J Pharmacol. 2016. PMID: 26756551 Free PMC article.
-
Cloning, expression and identification of KTX-Sp4, a selective Kv1.3 peptidic blocker from Scorpiops pococki.Cell Biosci. 2017 Nov 6;7:60. doi: 10.1186/s13578-017-0187-x. eCollection 2017. Cell Biosci. 2017. PMID: 29142737 Free PMC article.
-
Spatiotemporal control of necroptotic cell death and plasma membrane recruitment using engineered MLKL domains.Cell Death Discov. 2022 Nov 29;8(1):469. doi: 10.1038/s41420-022-01258-0. Cell Death Discov. 2022. PMID: 36446770 Free PMC article.
-
A Metabolomic Study of the Analgesic Effect of Lappaconitine Hydrobromide (LAH) on Inflammatory Pain.Metabolites. 2022 Sep 29;12(10):923. doi: 10.3390/metabo12100923. Metabolites. 2022. PMID: 36295824 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

