Evidence-based new service package vs. routine service package for smoking cessation to prevent high risk patients from cardiovascular diseases (CVD): study protocol for randomized controlled trial
- PMID: 24308874
- PMCID: PMC4028806
- DOI: 10.1186/1745-6215-14-419
Evidence-based new service package vs. routine service package for smoking cessation to prevent high risk patients from cardiovascular diseases (CVD): study protocol for randomized controlled trial
Abstract
Background: Smoking cessation is a high-priority intervention to prevent CVD events and deaths in developing countries. While several interventions to stop smoking have been proved successful, the question of how to increase their effectiveness and practicality in developing countries remains. In this study, a newly devised evidence-based smoking cessation service package will be compared with the existing service in a randomized controlled trial within the community setting of Thailand.
Method/design: This randomized control trial will recruit 440 current smokers at CVD risk because of being diabetic and/or hypertensive. Informed, consented participants will be randomly allocated into the new service-package arm and the routine service arm. The study will take place in the non-communicable disease clinics of the Maetha District Hospital, Lampang, northern Thailand. The new smoking-cessation service-package comprises (1) regular patient motivation and coaching from the same primary care nurse over a 3-month period; (2) monthly application of piCO + smokerlyzer to sustain motivation of smoker's quitting attempt and provide positive feedback over a 3-month period; (3) assistance by an assigned family member; (4) nicotine replacement chewing gum to relieve withdrawal symptoms. This new service will be compared with the traditional routine service comprising the 5A approach in a 1-year follow-up. Participants who consent to participate in the study but refuse to attempt quitting smoking will be allocated to the non-randomized arm, where they will be just followed up and monitored. Primary outcome of the study is smoking cessation rate at 1-year follow-up proven by breath analysis measuring carbomonoxide in parts per million in expired air. Secondary outcomes are smoking cessation rate at the 6-month follow-up, blood pressure and heart rate, CVD risk according to the Framingham general cardiovascular risk score, CVD events and deaths at the 12-month follow-up, and the cost-effectiveness of the health service packages. Intention-to-treat analysis will be followed. Factors influencing smoking cessation will be analyzed by the structure equation model.
Discussion: This multicomponent intervention, accessible at primary healthcare clinics, and focusing on the individual as well as the family and social environment, is unique and expected to work effectively.
Trial registration: Current Controlled Trials ISRCTN89315117.
Figures
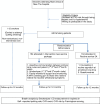

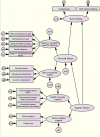

References
-
- WHO. Cardiovascular disease. http://www.who.int/cardiovascular_diseases/en/
-
- World Hlealth Organization. Global health observatory (GHO). Risk Factors. http://www.who.int/gho/ncd/risk_factors/en/index.html.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

