Unacylated ghrelin promotes skeletal muscle regeneration following hindlimb ischemia via SOD-2-mediated miR-221/222 expression
- PMID: 24308935
- PMCID: PMC3886736
- DOI: 10.1161/JAHA.113.000376
Unacylated ghrelin promotes skeletal muscle regeneration following hindlimb ischemia via SOD-2-mediated miR-221/222 expression
Abstract
Background: Surgical treatment of peripheral artery disease, even if successful, does not prevent reoccurrence. Under these conditions, increased oxidative stress is a crucial determinant of tissue damage. Given its reported antioxidant effects, we investigated the potential of unacylated-ghrelin (UnAG) to reduce ischemia-induced tissue damage in a mouse model of peripheral artery disease.
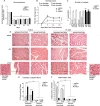
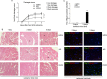
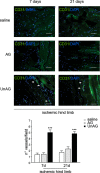
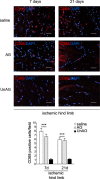
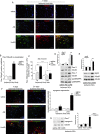
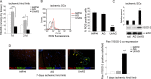
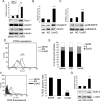
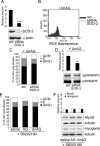
Methods and results: We show that UnAG but not acylated ghrelin (AG) induces skeletal muscle regeneration in response to ischemia via canonical p38/mitogen-actived protein kinase signaling UnAG protected against reactive oxygen species-induced cell injuries by inducing the expression of superoxide dismutase-2 (SOD-2) in satellite cells. This led to a reduced number of infiltrating CD68(+) cells and was followed by induction of the myogenic process and a reduction in functional impairment. Moreover, we found that miR-221/222, previously linked to muscle regeneration processes, was up-regulated and negatively correlated with p57(Kip2) expression in UnAG-treated mice. UnAG, unlike AG, promoted cell-cycle entry in satellite cells of mice lacking the genes for ghrelin and its receptor (GHSR1a). UnAG-induced p38/mitogen-actived protein kinase phosphorylation, leading to activation of the myogenic process, was prevented in SOD-2-depleted SCs. By siRNA technology, we also demonstrated that SOD-2 is the antioxidant enzyme involved in the control of miR-221/222-driven posttranscriptional p57(Kip2) regulation. Loss-of-function experiments targeting miR-221/222 and local pre-miR-221/222 injection in vivo confirmed a role for miR-221/222 in driving skeletal muscle regeneration after ischemia.
Conclusions: These results indicate that UnAG-induced skeletal muscle regeneration after ischemia depends on SOD-2-induced miR-221/222 expression and highlight its clinical potential for the treatment of reactive oxygen species-mediated skeletal muscle damage.
Keywords: ROS; UnAG; miRNAs; satellite cells; superoxide dismutase‐2.
Figures








Similar articles
-
Unacylated ghrelin induces oxidative stress resistance in a glucose intolerance and peripheral artery disease mouse model by restoring endothelial cell miR-126 expression.Diabetes. 2015 Apr;64(4):1370-82. doi: 10.2337/db14-0991. Epub 2014 Nov 3. Diabetes. 2015. PMID: 25368096
-
Unacylated Ghrelin Enhances Satellite Cell Function and Relieves the Dystrophic Phenotype in Duchenne Muscular Dystrophy mdx Model.Stem Cells. 2017 Jul;35(7):1733-1746. doi: 10.1002/stem.2632. Epub 2017 May 7. Stem Cells. 2017. PMID: 28436144
-
Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice.J Clin Invest. 2013 Feb;123(2):611-22. doi: 10.1172/JCI39920. Epub 2013 Jan 2. J Clin Invest. 2013. PMID: 23281394 Free PMC article.
-
Unlocking the Potential of Obestatin: A Novel Peptide Intervention for Skeletal Muscle Regeneration and Prevention of Atrophy.Mol Biotechnol. 2024 May;66(5):948-959. doi: 10.1007/s12033-023-01011-7. Epub 2024 Jan 10. Mol Biotechnol. 2024. PMID: 38198052 Review.
-
MicroRNAs in the regeneration of skeletal muscle.Front Biosci (Landmark Ed). 2013 Jan 1;18(2):608-15. doi: 10.2741/4124. Front Biosci (Landmark Ed). 2013. PMID: 23276946 Review.
Cited by
-
Both ghrelin deletion and unacylated ghrelin overexpression preserve muscles in aging mice.Aging (Albany NY). 2020 Jul 26;12(14):13939-13957. doi: 10.18632/aging.103802. Epub 2020 Jul 26. Aging (Albany NY). 2020. PMID: 32712599 Free PMC article.
-
Antifibrotic activity of acylated and unacylated ghrelin.Int J Endocrinol. 2015;2015:385682. doi: 10.1155/2015/385682. Epub 2015 Apr 16. Int J Endocrinol. 2015. PMID: 25960743 Free PMC article. Review.
-
MiR-222 in Cardiovascular Diseases: Physiology and Pathology.Biomed Res Int. 2017;2017:4962426. doi: 10.1155/2017/4962426. Epub 2017 Jan 3. Biomed Res Int. 2017. PMID: 28127557 Free PMC article. Review.
-
Ghrelin improves functional survival of engrafted adipose-derived mesenchymal stem cells in ischemic heart through PI3K/Akt signaling pathway.Biomed Res Int. 2015;2015:858349. doi: 10.1155/2015/858349. Epub 2015 Mar 24. Biomed Res Int. 2015. PMID: 25879037 Free PMC article.
-
Acylated Ghrelin Receptor Agonist HM01 Decreases Lean Body and Muscle Mass, but Unacylated Ghrelin Protects against Redox-Dependent Sarcopenia.Antioxidants (Basel). 2022 Nov 28;11(12):2358. doi: 10.3390/antiox11122358. Antioxidants (Basel). 2022. PMID: 36552566 Free PMC article.
References
-
- Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Jr, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996; 94:3026-3049 - PubMed
-
- Alexander CM, Landsman PB, Teutsch SM, Haffner SMThird National Health and Nutrition Examination Survey (NHANES III); National Cholesterol Education Program (NCEP) NCEP‐defined metabolic syndrome, diabetes, and prevalence of coronary heart disease among NHANES III participants age 50 years and older. Diabetes. 2003; 52:1210-1214 - PubMed
-
- Gray BH, Conte MS, Dake MD, Jaff MR, Kandarpa K, Ramee SR, Rundback J, Waksman RAmerican Heart Association Writing Group 7 Atherosclerotic Peripheral Vascular Disease Symposium II: lower‐extremity revascularization: state of the art. Circulation. 2008; 118:2864-2872 - PubMed
-
- Cieri E, Lenti M, De Rango P, Isernia G, Marucchini A, Cao P. Functional ability in patients with critical limb ischaemia is unaffected by successful revascularisation. Eur J Vasc Endovasc Surg. 2011; 41:256-263 - PubMed
-
- Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vasc Endovascular Surg. 2008; 42:101-112 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

