Mechanical dyssynchrony precedes QRS widening in ATP-sensitive K⁺ channel-deficient dilated cardiomyopathy
- PMID: 24308936
- PMCID: PMC3886734
- DOI: 10.1161/JAHA.113.000410
Mechanical dyssynchrony precedes QRS widening in ATP-sensitive K⁺ channel-deficient dilated cardiomyopathy
Abstract
Background: Contractile discordance exacerbates cardiac dysfunction, aggravating heart failure outcome. Dissecting the genesis of mechanical dyssynchrony would enable an early diagnosis before advanced disease.
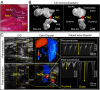
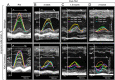
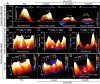
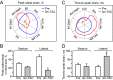
Methods and results: High-resolution speckle-tracking echocardiography was applied in a knockout murine surrogate of adult-onset human cardiomyopathy caused by mutations in cardioprotective ATP-sensitive K(+) (K(ATP)) channels. Preceding the established criteria of cardiac dyssynchrony, multiparametric speckle-based strain resolved nascent erosion of dysfunctional regions within cardiomyopathic ventricles of the K(ATP) channel-null mutant exposed to hemodynamic stress. Not observed in wild-type counterparts, intraventricular disparity in wall motion, validated by the degree, direction, and delay of myocardial speckle patterns, unmasked the disease substrate from asymptomatic to overt heart failure. Mechanical dyssynchrony preceded widening of the QRS complex and exercise intolerance and progressed into global myocardial discoordination and decompensated cardiac pump function, precipitating a low output syndrome.
Conclusions: The present study, with the use of high-resolution imaging, prospectively resolved the origin and extent of intraventricular motion disparity in a K(ATP) channel-knockout model of dilated cardiomyopathy. Mechanical dyssynchrony established as an early marker of cardiomyopathic disease offers novel insight into the pathodynamics of dyssynchronous heart failure.
Keywords: ATP‐sensitive K+ channel; Kir6.2; QRS complex; heart failure; speckle‐tracking.
Figures



References
-
- Watkins H, Ashrafian H, Redwood C. Inherited cardiomyopathies. N Engl J Med. 2011; 364:1643-1656 - PubMed
-
- Piran S, Liu P, Morales A, Hershberger RE. Where genome meets phenome: rationale for integrating genetic and protein biomarkers in the diagnosis and management of dilated cardiomyopathy and heart failure. J Am Coll Cardiol. 2012; 60:283-289 - PubMed
-
- ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012. Eur Heart J. 2012; 33:1787-1847 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

