Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates
- PMID: 24308974
- PMCID: PMC3929681
- DOI: 10.1096/fj.13-241497
Rapid evolution of binding specificities and expression patterns of inhibitory CD33-related Siglecs in primates
Abstract
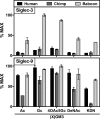
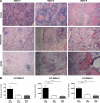
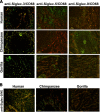
Siglecs are sialic acid-binding Ig-like lectins that recognize sialoglycans via amino-terminal V-set domains. CD33-related Siglecs (CD33rSiglecs) on innate immune cells recognize endogenous sialoglycans as "self-associated molecular patterns" (SAMPs), dampening immune responses via cytosolic immunoreceptor tyrosine-based inhibition motifs that recruit tyrosine phosphatases. However, sialic acid-expressing pathogens subvert this mechanism through molecular mimicry. Meanwhile, endogenous host SAMPs must continually evolve to evade other pathogens that exploit sialic acids as invasion targets. We hypothesized that these opposing selection forces have accelerated CD33rSiglec evolution. We address this by comparative analysis of major CD33rSiglec (Siglec-3, Siglec-5, and Siglec-9) orthologs in humans, chimpanzees, and baboons. Recombinant soluble molecules displaying ligand-binding domains show marked quantitative and qualitative interspecies differences in interactions with strains of the sialylated pathogen, group B Streptococcus, and with sialoglycans presented as gangliosides or in the form of sialoglycan microarrays, including variations such as N-glycolyl and O-acetyl groups. Primate Siglecs also show quantitative and qualitative intra- and interspecies variations in expression patterns on leukocytes, both in circulation and in tissues. Taken together our data explain why the CD33rSiglec-encoding gene cluster is undergoing rapid evolution via multiple mechanisms, driven by the need to maintain self-recognition by innate immune cells, while escaping 2 distinct mechanisms of pathogen subversion.
Keywords: N-acetylneuraminic acid; N-glycolylneuraminic acid; innate immunity; sialic acids.
Figures




Similar articles
-
Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation?Immunology. 2011 Jan;132(1):18-26. doi: 10.1111/j.1365-2567.2010.03368.x. Epub 2010 Nov 11. Immunology. 2011. PMID: 21070233 Free PMC article. Review.
-
Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms.Proc Natl Acad Sci U S A. 2004 Sep 7;101(36):13251-6. doi: 10.1073/pnas.0404833101. Epub 2004 Aug 26. Proc Natl Acad Sci U S A. 2004. PMID: 15331780 Free PMC article.
-
Cloning, characterization, and phylogenetic analysis of siglec-9, a new member of the CD33-related group of siglecs. Evidence for co-evolution with sialic acid synthesis pathways.J Biol Chem. 2000 Jul 21;275(29):22127-35. doi: 10.1074/jbc.M002775200. J Biol Chem. 2000. PMID: 10801860
-
Sialic acid-binding immunoglobulin-like lectins (Siglecs) detect self-associated molecular patterns to regulate immune responses.Cell Mol Life Sci. 2020 Feb;77(4):593-605. doi: 10.1007/s00018-019-03288-x. Epub 2019 Sep 4. Cell Mol Life Sci. 2020. PMID: 31485715 Free PMC article. Review.
-
Discovery, classification, evolution and diversity of Siglecs.Mol Aspects Med. 2023 Apr;90:101117. doi: 10.1016/j.mam.2022.101117. Epub 2022 Aug 18. Mol Aspects Med. 2023. PMID: 35989204 Free PMC article. Review.
Cited by
-
Protein transduction therapy into cochleae via the round window niche in guinea pigs.Mol Ther Methods Clin Dev. 2016 Aug 17;3:16055. doi: 10.1038/mtm.2016.55. eCollection 2016. Mol Ther Methods Clin Dev. 2016. PMID: 27579336 Free PMC article.
-
Comparative physiological anthropogeny: exploring molecular underpinnings of distinctly human phenotypes.Physiol Rev. 2023 Jul 1;103(3):2171-2229. doi: 10.1152/physrev.00040.2021. Epub 2023 Jan 5. Physiol Rev. 2023. PMID: 36603157 Free PMC article. Review.
-
Neuroimmune multi-hit perspective of coronaviral infection.J Neuroinflammation. 2021 Oct 13;18(1):231. doi: 10.1186/s12974-021-02282-0. J Neuroinflammation. 2021. PMID: 34645457 Free PMC article. Review.
-
A versatile soluble siglec scaffold for sensitive and quantitative detection of glycan ligands.Nat Commun. 2020 Oct 9;11(1):5091. doi: 10.1038/s41467-020-18907-6. Nat Commun. 2020. PMID: 33037195 Free PMC article.
-
Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus.J Exp Med. 2014 Jun 2;211(6):1231-42. doi: 10.1084/jem.20131853. Epub 2014 May 5. J Exp Med. 2014. PMID: 24799499 Free PMC article.
References
-
- Angata T., Varki A. (2002) Chemical diversity in the sialic acids and related α-keto acids: an evolutionary perspective. Chem. Rev. 102, 439–469 - PubMed
-
- Crocker P. R., Paulson J. C., Varki A. (2007) Siglecs and their roles in the immune system. Nat. Rev. Immunol. 7, 255–266 - PubMed
-
- Kelm S., Pelz A., Schauer R., Filbin M. T., Tang S., De Bellard M.-E., Schnaar R. L., Mahoney J. A., Hartnell A., Bradfield P., Crocker P. R. (1994) Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr. Biol. 4, 965–972 - PubMed
-
- Powell L. D., Varki A. (1995) I-type lectins. J. Biol. Chem. 270, 14243–14246 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

