Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation
- PMID: 24309208
- PMCID: PMC3933391
- DOI: 10.1016/j.ydbio.2013.11.023
Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation
Abstract
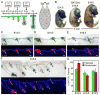
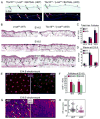
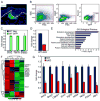
Broad dermal Wnt signaling is required for patterned induction of hair follicle placodes and subsequent Wnt signaling in placode stem cells is essential for induction of dermal condensates, cell clusters of precursors for the hair follicle dermal papilla (DP). Progression of hair follicle formation then requires coordinated signal exchange between dermal condensates and placode stem cells. However, it remains unknown whether continued Wnt signaling in DP precursor cells plays a role in this process, largely due to the long-standing inability to specifically target dermal condensates for gene ablation. Here we use the Tbx18(Cre) knockin mouse line to ablate the Wnt-responsive transcription factor β-catenin specifically in these cells at E14.5 during the first wave of guard hair follicle formation. In the absence of β-catenin, canonical Wnt signaling is effectively abolished in these cells. Sox2(+) dermal condensates initiate normally; however by E16.5 guard hair follicle numbers are strongly reduced and by E18.5 most whiskers and guard hair follicles are absent, suggesting that active Wnt signaling in dermal condensates is important for hair follicle formation to proceed after induction. To explore the molecular mechanisms by which Wnt signaling in dermal condensates regulates hair follicle formation, we analyze genome-wide the gene expression changes in embryonic β-catenin null DP precursor cells. We find altered expression of several signaling pathway genes, including Fgfs and Activin, both previously implicated in hair follicle formation. In summary, these data reveal a functional role of Wnt signaling in DP precursors for embryonic hair follicle formation and identify Fgf and Activin signaling as potential effectors of Wnt signaling-regulated events.
Keywords: Dermal papilla cells; Hair follicle morphogenesis; Hair follicle stem cells; Stem cell niche; Wnt signaling.
© 2013 Published by Elsevier Inc.
Figures


References
-
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. - PubMed
-
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev Cell. 2002;2:643–653. - PubMed
-
- Botchkarev VA, Botchkareva NV, Roth W, Nakamura M, Chen LH, Herzog W, Lindner G, McMahon JA, Peters C, Lauster R, McMahon AP, Paus R. Noggin is a mesenchymally derived stimulator of hair-follicle induction. Nat Cell Biol. 1999;1:158–164. - PubMed
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
-
- Brecht M, Preilowski B, Merzenich MM. Functional architecture of the mystacial vibrissae. Behav Brain Res. 1997;84:81–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

