Neuronal merlin influences ERBB2 receptor expression on Schwann cells through neuregulin 1 type III signalling
- PMID: 24309211
- PMCID: PMC3914471
- DOI: 10.1093/brain/awt327
Neuronal merlin influences ERBB2 receptor expression on Schwann cells through neuregulin 1 type III signalling
Abstract
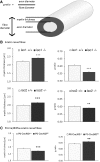
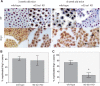
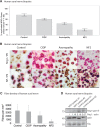
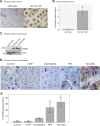
Axonal surface proteins encompass a group of heterogeneous molecules, which exert a variety of different functions in the highly interdependent relationship between axons and Schwann cells. We recently revealed that the tumour suppressor protein merlin, mutated in the hereditary tumour syndrome neurofibromatosis type 2, impacts significantly on axon structure maintenance in the peripheral nervous system. We now report on a role of neuronal merlin in the regulation of the axonal surface protein neuregulin 1 important for modulating Schwann cell differentiation and myelination. Specifically, neuregulin 1 type III expression is reduced in sciatic nerve tissue of neuron-specific knockout animals as well as in biopsies from seven patients with neurofibromatosis type 2. In vitro experiments performed on both the P19 neuronal cell line and primary dorsal root ganglion cells demonstrate the influence of merlin on neuregulin 1 type III expression. Moreover, expression of ERBB2, a Schwann cell receptor for neuregulin 1 ligands is increased in nerve tissue of both neuron-specific merlin knockout animals and patients with neurofibromatosis type 2, demonstrating for the first time that axonal merlin indirectly regulates Schwann cell behaviour. Collectively, we have identified that neuronally expressed merlin can influence Schwann cell activity in a cell-extrinsic manner.
Keywords: Schwann cell; axon; merlin; myelination; neuregulin 1; neurofibromatosis type 2; polyneuropathy.
Figures



References
-
- Baser MER, Evans DG, Gutmann DH. Neurofibromatosis 2. Curr Opin Neurol. 2003;16:27–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

